Drug Catalog - Product Detail
ALFUZOSIN HCL ER TB 10MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 69097-0844-07 | CIPLA USA | 100 | 10MG | TABLET |
PACKAGE FILES

Generic Name
ALFUZOSIN HYDROCHLORIDE
Substance Name
ALFUZOSIN HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090284
Description
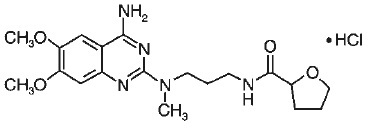
11 DESCRIPTION Each alfuzosin hydrochloride extended-release tablets, USP contains 10 mg alfuzosin hydrochloride as the active ingredient. Alfuzosin hydrochloride is a white to off-white crystalline powder that melts at approximately 240°C. It is freely soluble in water, sparingly soluble in alcohol, and practically insoluble in dichloromethane. Alfuzosin hydrochloride, USP is (R, S)-N-[3-[(4-amino-6, 7-dimethoxy-2-quinazolinyl) methylamino] propyl] tetrahydro-2-furancarboxamide hydrochloride. The empirical formula of alfuzosin hydrochloride is C 19 H 27 N 5 O 4 •HCI. The molecular weight of alfuzosin hydrochloride is 425.9. Its structural formula is: The tablet also contains the following inactive ingredients: microcrystalline cellulose (NF), guar gum (NF), hydroxypropyl methyl cellulose (USP), colloidal silicon dioxide (NF) and magnesium stearate (NF). Meets USP Dissolution Test 4. Image
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Alfuzosin Hydrochloride Extended-Release Tablets, USP 10 mg are available as off white, round, biconvex tablets debossed with 'IG' on one side and "302" on other. Alfuzosin Hydrochloride Extended-Release Tablets are supplied as follows: Package NDC Number Bottles of 30 69097-844-02 Bottles of 90 69097-844-05 Bottles of 100 69097-844-07 Bottles of 500 69097-844-12 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container as defined in the USP. Keep alfuzosin hydrochloride out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Alfuzosin hydrochloride extended-release tablet USP, is an alpha adrenergic antagonist, indicated for treatment of signs and symptoms of benign prostatic hyperplasia. ( 1 ) Important Limitations of Use: Alfuzosin hydrochloride is not indicated for the treatment of hypertension. ( 1.1 ) Alfuzosin hydrochloride is not indicated for use in the pediatric population. ( 1.1 , 8.4 , 12.3 ) Alfuzosin hydrochloride tablets USP, are indicated for the treatment of signs and symptoms of benign prostatic hyperplasia. 1.1 Important Limitations of Use Alfuzosin hydrochloride is not indicated for the treatment of hypertension. Alfuzosin hydrochloride is not indicated for use in the pediatric population.
Dosage and Administration
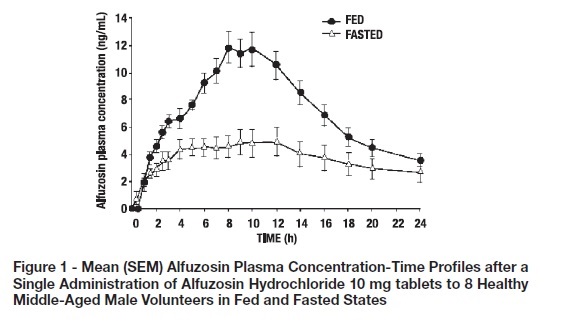
2 DOSAGE AND ADMINISTRATION 10 mg once daily with food and with the same meal each day ( 2 ) Tablets should not be chewed or crushed ( 2 , 12.3 ) The recommended dosage is one 10 mg alfuzosin hydrochloride extended-release tablet once daily. The extent of absorption of alfuzosin is 50% lower under fasting conditions. Therefore, alfuzosin hydrochloride should be taken with food and with the same meal each day. The tablets should not be chewed or crushed.
