Drug Catalog - Product Detail
ALLOPURINOL USP TB 100MG 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-5543-10 | ACTAVIS PHARMA | 1000 | 100MG | TABLET |
PACKAGE FILES


Generic Name
ALLOPURINOL
Substance Name
ALLOPURINOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA018877
Description
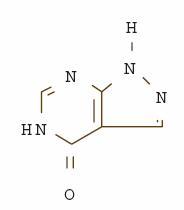
DESCRIPTION Allopurinol, USP is known chemically as 1,5-dihydro-4 H -pyrazolo [3,4- d ]pyrimidin-4-one. It is a xanthine oxidase inhibitor which is administered orally. Its solubility in water at 37°C is 80.0 mg/dL and is greater in an alkaline solution. The structural formula is represented below: C 5 H 4 N 4 O M.W. 136.11 Allopurinol Tablets USP, 100 mg and 300 mg contain the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized corn starch and sodium lauryl sulfate. Allopurinol Tablets USP, 300 mg also contain FD&C Yellow No. 6. Allopurinol structural formula
How Supplied
HOW SUPPLIED Allopurinol Tablets USP, 100 mg are scored, round, white tablets imprinted “ DAN DAN” and “ 5543” supplied in bottles of 100 (NDC 0591-5543-01) and 1000 (NDC 0591-5543-10). Allopurinol Tablets USP, 300 mg are scored, round, orange tablets imprinted “ DAN DAN” and “ 5544” supplied in bottles of 100 (NDC 0591-5544-01) and 500 (NDC 0591-5544-05). Dispense in a tight, light-resistant container with child-resistant closure. Store at 20 to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light. Manufactured In India By: Watson Pharma Private Limited Verna, Salcette Goa 403 722 INDIA Distributed By: Actavis Pharma, Inc. Parsippany, NJ 07054 USA Rev. A 1/2019
Indications & Usage
INDICATIONS AND USAGE THIS IS NOT AN INNOCUOUS DRUG. IT IS NOT RECOMMENDED FOR THE TREATMENT OF ASYMPTOMATIC HYPERURICEMIA. Allopurinol reduces serum and urinary uric acid concentrations. Its use should be individualized for each patient and requires an understanding of its mode of action and pharmacokinetics (see CLINICAL PHARMACOLOGY , CONTRAINDICATIONS , WARNINGS , and PRECAUTIONS ). Allopurinol is indicated in: the management of patients with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or nephropathy). the management of patients with leukemia, lymphoma and malignancies who are receiving cancer therapy which causes elevations of serum and urinary uric acid levels. Treatment with allopurinol should be discontinued when the potential for overproduction of uric acid is no longer present. the management of patients with recurrent calcium oxalate calculi whose daily uric acid excretion exceeds 800 mg/day in male patients and 750 mg/day in female patients. Therapy in such patients should be carefully assessed initially and reassessed periodically to determine in each case that treatment is beneficial and that the benefits outweigh the risks.
Dosage and Administration
DOSAGE AND ADMINISTRATION The dosage of allopurinol to accomplish full control of gout and to lower serum uric acid to normal or near-normal levels varies with the severity of the disease. The average is 200 to 300 mg/day for patients with mild gout and 400 to 600 mg/day for those with moderately severe tophaceous gout. The appropriate dosage may be administered in divided doses or as a single equivalent dose with the 300 mg tablet. Dosage requirements in excess of 300 mg should be administered in divided doses. The minimal effective dosage is 100 to 200 mg daily and the maximal recommended dosage is 800 mg daily. To reduce the possibility of flare-up of acute gouty attacks, it is recommended that the patient start with a low dose of allopurinol (100 mg daily) and increase at weekly intervals by 100 mg until a serum uric acid level of 6 mg/dL or less is attained but without exceeding the maximal recommended dosage. Normal serum urate levels are usually achieved in 1 to 3 weeks. The upper limit of normal is about 7 mg/dL for men and postmenopausal women and 6 mg/dL for premenopausal women. Too much reliance should not be placed on a single serum uric acid determination since, for technical reasons, estimation of uric acid may be difficult. By selecting the appropriate dosage and, in certain patients, using uricosuric agents concurrently, it is possible to reduce serum uric acid to normal or, if desired, to as low as 2 to 3 mg/dL and keep it there indefinitely. While adjusting the dosage of allopurinol in patients who are being treated with colchicine and/or anti-inflammatory agents, it is wise to continue the latter therapy until serum uric acid has been normalized and there has been freedom from acute gouty attacks for several months. In transferring a patient from a uricosuric agent to allopurinol, the dose of the uricosuric agent should be gradually reduced over a period of several weeks and the dose of allopurinol gradually increased to the required dose needed to maintain a normal serum uric acid level. It should also be noted that allopurinol is generally better tolerated if taken following meals. A fluid intake sufficient to yield a daily urinary output of at least 2 liters and the maintenance of a neutral or preferably, slightly alkaline urine are desirable. Since allopurinol and its metabolites are primarily eliminated only by the kidney, accumulation of the drug can occur in renal failure, and the dose of allopurinol should consequently be reduced. With a creatinine clearance of 10 to 20 mL/min, a daily dosage of 200 mg of allopurinol is suitable. When the creatinine clearance is less than 10 mL/min, the daily dosage should not exceed 100 mg. With extreme renal impairment (creatinine clearance less than 3 mL/min) the interval between doses may also need to be lengthened. The correct size and frequency of dosage for maintaining the serum uric acid just within the normal range is best determined by using the serum uric acid level as an index. For the prevention of uric acid nephropathy during the vigorous therapy of neoplastic disease, treatment with 600 to 800 mg daily for 2 or 3 days is advisable together with a high fluid intake. Otherwise similar considerations to the above recommendations for treating patients with gout govern the regulation of dosage for maintenance purposes in secondary hyperuricemia. The dose of allopurinol recommended for management of recurrent calcium oxalate stones in hyperuricosuric patients is 200 to 300 mg/day in divided doses or as the single equivalent. This dose may be adjusted up or down depending upon the resultant control of the hyperuricosuria based upon subsequent 24 hour urinary urate determinations. Clinical experience suggests that patients with recurrent calcium oxalate stones may also benefit from dietary changes such as the reduction of animal protein, sodium, refined sugars, oxalate-rich foods, and excessive calcium intake, as well as an increase in oral fluids and dietary fiber. Children, 6 to 10 years of age, with secondary hyperuricemia associated with malignancies may be given 300 mg allopurinol daily while those under 6 years are generally given 150 mg daily. The response is evaluated after approximately 48 hours of therapy and a dosage adjustment is made if necessary.
