Drug Catalog - Product Detail
AMBRISENTAN 10MG FC TABLETS 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-2406-30 | ACTAVIS PHARMA | 30 | 10MG | TABLET |
PACKAGE FILES








Generic Name
AMBRISENTAN
Substance Name
AMBRISENTAN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA208252
Description
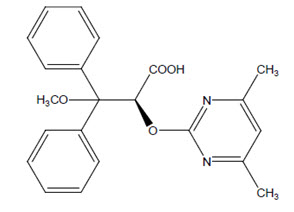
11 DESCRIPTION Ambrisentan is an endothelin receptor antagonist that is selective for the endothelin type-A (ET A ) receptor. The chemical name of ambrisentan is (+)-(2 S )-2-[(4,6-dimethylpyrimidin-2-yl)oxy]-3-methoxy-3,3-diphenylpropanoic acid. It has a molecular formula of C 22 H 22 N 2 O 4 and a molecular weight of 378.42. It contains a single chiral center determined to be the ( S ) configuration and has the following structural formula: Figure 1: Ambrisentan Structural Formula Ambrisentan is a white to off-white, crystalline solid. It is a carboxylic acid with a pKa of 4.0. Ambrisentan is practically insoluble in water and in aqueous solutions at low pH. Solubility increases in aqueous solutions at higher pH. In the solid state ambrisentan is very stable, is not hygroscopic, and is not light sensitive. Ambrisentan is available as 5 mg and 10 mg film-coated tablets for once daily oral administration. The tablets include the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing lecithin, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. Each 5 mg ambrisentan film-coated tablet is white to off-white and capsule shaped. Each 10 mg ambrisentan film-coated tablet is white to off-white and capsule shaped. Ambrisentan tablets are unscored. Figure 1 Ambrisentan Structural Formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Ambrisentan film - coated tablets are supplied as follows: Tablet Strength Package Configuration NDC No. Description of Tablet; Debossed on Tablet; Size 5 mg 30 count bottle 0591 - 2405 - 30 White to off-white; capsule shaped; film- coated tablet; debossed with "5" on one side and "405" on the other side; 8.0 mm x 4.0 mm 10 mg 30 count bottle 0591 - 2406 - 30 White to off-white; capsule shaped; film- coated tablet; debossed with " 10 " on one side and "406" on the other side; 11.00 mm x 5.10 mm Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Store ambrisentan tablets in its original packaging.
Indications & Usage
1 INDICATIONS AND USAGE Ambrisentan tablets are indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1): To improve exercise ability and delay clinical worsening. In combination with tadalafil to reduce the risks of disease progression and hospitalization for worsening PAH, and to improve exercise ability [see Clinical Studies ( 14.2 )] . Studies establishing effectiveness included predominantly patients with WHO Functional Class II–III symptoms and etiologies of idiopathic or heritable PAH (60%) or PAH associated with connective tissue diseases (34%). Ambrisentan is an endothelin receptor antagonist indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1): To improve exercise ability and delay clinical worsening. In combination with tadalafil to reduce the risks of disease progression and hospitalization for worsening PAH, and to improve exercise ability. Studies establishing effectiveness included trials predominantly in patients with WHO Functional Class II–III symptoms and etiologies of idiopathic or heritable PAH (60%) or PAH associated with connective tissue diseases (34%) ( 1 ).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Initiate treatment at 5 mg once daily ( 2.1 ). May be started with tadalafil ( 2.1 ). Titrate at 4-week intervals as needed and tolerated ( 2.1 ). Do not split, crush, or chew tablets ( 2.1 ). 2.1 Adult Dosage Initiate treatment at 5 mg once daily, with or without tadalafil 20 mg once daily. At 4-week intervals, either the dose of ambrisentan or tadalafil can be increased, as needed and tolerated, to ambrisentan 10 mg or tadalafil 40 mg. Do not split, crush, or chew tablets. 2.2 Pregnancy Testing in Females of Reproductive Potential Initiate treatment with ambrisentan in females of reproductive potential only after a negative pregnancy test. Obtain monthly pregnancy tests during treatment [see Use in Specific Populations ( 8.3 )].
