Drug Catalog - Product Detail
AMITRIPTYLINE HCL TB 10MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16729-0171-01 | ACCORD HEALTHCARE | 100 | 10MG | TABLET |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
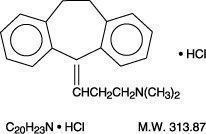
DESCRIPTION Amitriptyline HCl, a dibenzocycloheptadiene derivative, is a white, or practically white, odorless, crystalline compound which is freely soluble in water and alcohol. It is designated chemically as 10,11-Dihydro-N,N-dimethyl-5 H -dibenzo[a,d] cycloheptene-Δ 5 , γ-propylamine hydrochloride. It has the following structural formula: Each tablet for oral administration contains 10, 25, 50, 75, 100, or 150 mg amitriptyline hydrochloride. Inactive ingredients include colloidal anhydrous silica, croscarmellose sodium, lactose (monohydrate), lecithin, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, iron oxide red (10, 50, 75, 100, or 150 mg),talc, titanium dioxide and xanthan gum. amitriptyline HCl chemical structure
How Supplied
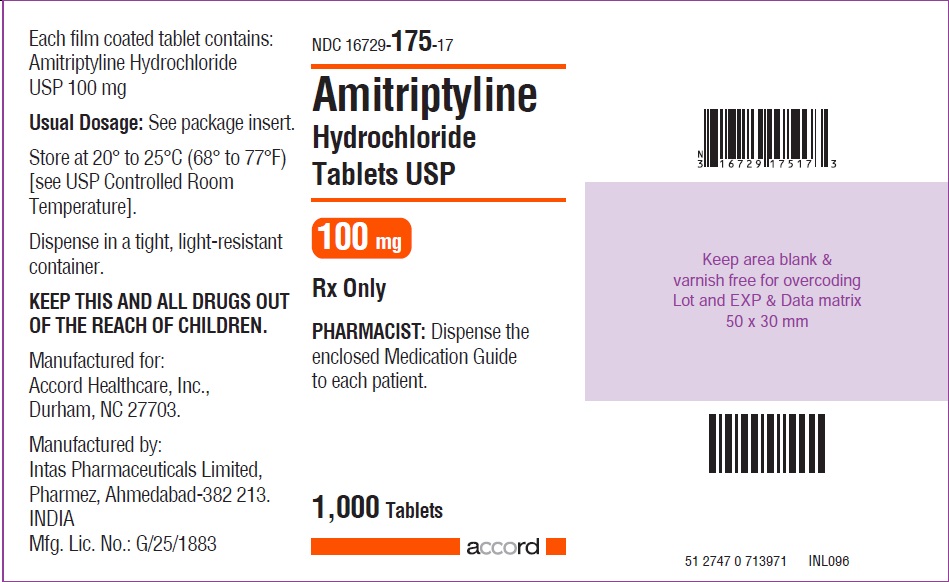
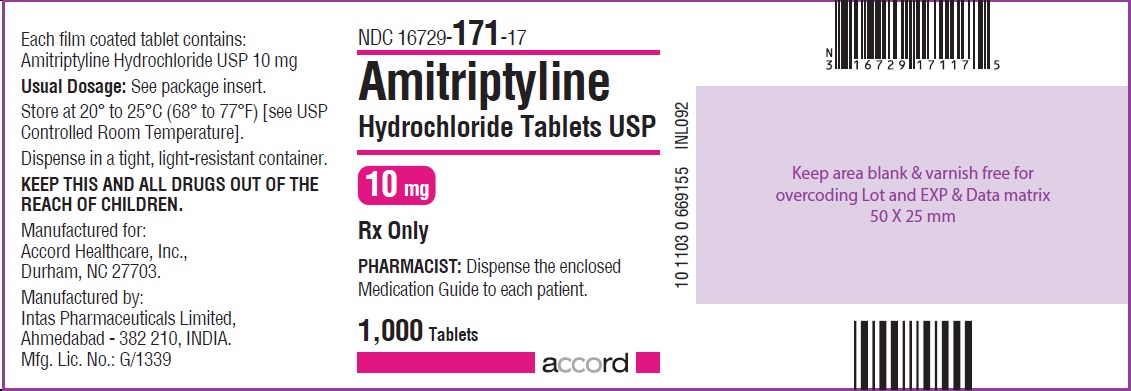
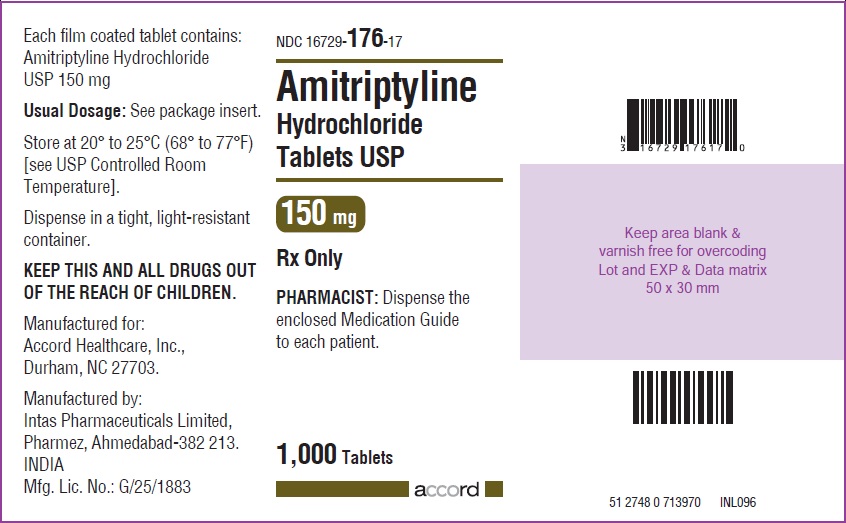
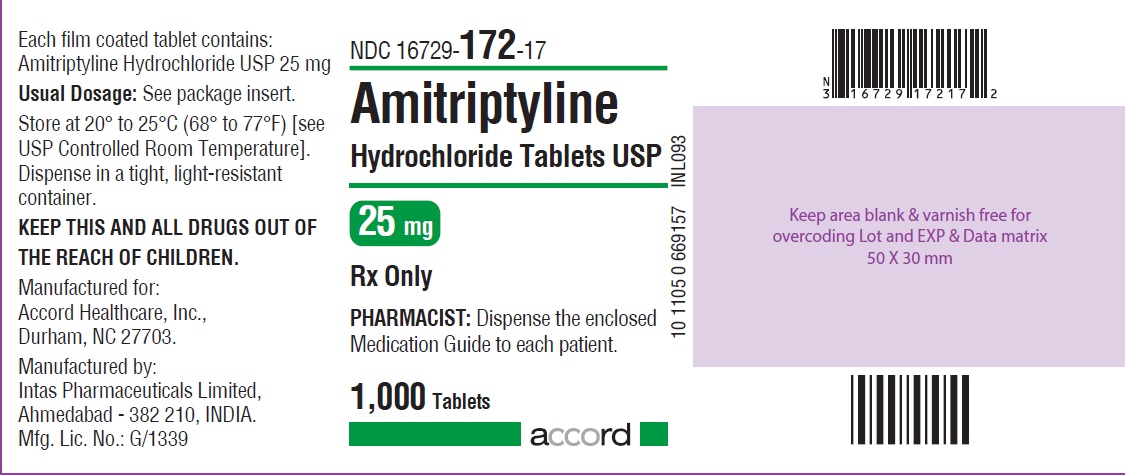
HOW SUPPLIED Amitriptyline hydrochloride tablets, USP for oral administration are available as: 10 mg: Brown coloured, round, biconvex, film coated tablet debossed with “I1” on one side and plain on other the side, and supplied as: NDC 16729-171-10 bottles of 30 NDC 16729-171-01 bottles of 100 NDC 16729-171-17 bottles of 1,000 25 mg: White coloured, round, biconvex, film coated tablet debossed with “NS” on one side and plain on the other side, and supplied as: NDC 16729-647-10 bottles of 30 NDC 16729-647-01 bottles of 100 NDC 16729-647-17 bottles of 1,000 50 mg: Brown coloured, round, biconvex, film coated tablet debossed with “NS1” on one side and plain on the other side, and supplied as: NDC 16729-648-10 bottles of 30 NDC 16729-648-01 bottles of 100 NDC 16729-648-17 bottles of 1,000 75 mg: Brown coloured, round, biconvex, film coated tablet debossed with “NS2” on one side and plain on the other side, and supplied as: NDC 16729-649-10 bottles of 30 NDC 16729-649-01 bottles of 100 NDC 16729-649-17 bottles of 1,000 100 mg: Brown coloured, round, biconvex, film coated tablet debossed with “NS3” on one side and plain on the other side, and supplied as: NDC 16729-650-10 bottles of 30 NDC 16729-650-01 bottles of 100 NDC 16729-650-17 bottles of 1,000 150 mg: Brown coloured, capsule shaped, biconvex, film coated tablet debossed with “I6” on one side and plain on the other side, and supplied as: NDC 16729-176-10 bottles of 30 NDC 16729-176-01 bottles of 100 NDC 16729-176-17 bottles of 1,000 Store at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container.
Indications & Usage
INDICATIONS AND USAGE For the relief of symptoms of depression. Endogenous depression is more likely to be alleviated than are other depressive states.
Dosage and Administration
DOSAGE AND ADMINISTRATION Oral Dosage Dosage should be initiated at a low level and increased gradually, noting carefully the clinical response and any evidence of intolerance. Initial Dosage for Adults For outpatients, 75 mg of amitriptyline HCl a day in divided doses is usually satisfactory. If necessary, this may be increased to a total of 150 mg per day. Increases are made preferably in the late afternoon and/or bedtime doses. A sedative effect may be apparent before the antidepressant effect is noted, but an adequate therapeutic effect may take as long as 30 days to develop. An alternate method of initiating therapy in outpatients is to begin with 50 to 100 mg amitriptyline HCl at bedtime. This may be increased by 25 or 50 mg as necessary in the bedtime dose to a total of 150 mg per day. Hospitalized patients may require 100 mg a day initially. This can be increased gradually to 200 mg a day if necessary. A small number of hospitalized patients may need as much as 300 mg a day. Adolescent and Elderly Patients In general, lower dosages are recommended for these patients. Ten mg 3 times a day with 20 mg at bedtime may be satisfactory in adolescent and elderly patients who do not tolerate higher dosages. Maintenance The usual maintenance dosage of amitriptyline HCl is 50 to 100 mg per day. In some patients, 40 mg per day is sufficient. For maintenance therapy, the total daily dosage may be given in a single dose, preferably at bedtime. When satisfactory improvement has been reached, dosage should be reduced to the lowest amount that will maintain relief of symptoms. It is appropriate to continue maintenance therapy 3 months or longer to lessen the possibility of relapse. Usage in Pediatric Patients In view of the lack of experience with the use of this drug in pediatric patients, it is not recommended at the present time for patients under 12 years of age. Plasma Levels Because of the wide variation in the absorption and distribution of tricyclic antidepressants in body fluids, it is difficult to directly correlate plasma levels and therapeutic effect. However, determination of plasma levels may be useful in identifying patients who appear to have toxic effects and may have excessively high levels, or those in whom lack of absorption or noncompliance is suspected. Because of increased intestinal transit time and decreased hepatic metabolism in elderly patients, plasma levels are generally higher for a given oral dose of amitriptyline hydrochloride than in younger patients. Elderly patients should be monitored carefully and quantitative serum levels obtained as clinically appropriate. Adjustments in dosage should be made according to the patient’s clinical response and not on the basis of plasma levels. Hollister, L.E.; Monitoring Tricyclic Antidepressant Plasma Concentrations. JAMA 1979; 241(23):2530-2533.
