Drug Catalog - Product Detail
ARIPIPRAZOLE ODT TB 10MG 3X10 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 62332-0103-30 | ALEMBIC PHARMACEUTICALS | 30 | 10MG | TABLET |
PACKAGE FILES




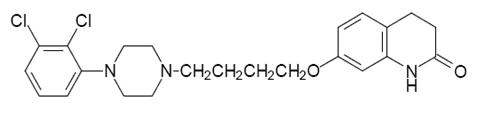
Generic Name
ARIPIPRAZOLE
Substance Name
ARIPIPRAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA202102
Description
11 DESCRIPTION Aripiprazole is an atypical antipsychotic drug that is available as Aripiprazole Orally Disintegrating Tablets. Aripiprazole is 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril. The empirical formula is C 23 H 27 Cl 2 N 3 O 2 and its molecular weight is 448.39. The chemical structure is: Aripiprazole Orally Disintegrating Tablets, USP are available in 10 mg and 15 mg strengths. Inactive ingredients include mannitol, aspartame, calcium stearate, crospovidone, vanilla, silicified microcrystalline cellulose, and lactose monohydrate. Colorants include iron oxide red. Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Aripiprazole Orally Disintegrating Tablets, USP are round tablets with markings on one side and plain on other side. Aripiprazole Orally Disintegrating Tablet is available in the strengths and packages listed in Table 33. Table 33: Aripiprazole Orally Disintegrating Tablets, USP Presentations Tablet Strength Tablet Color Tablet Markings Pack Size NDC Code 10 mg light pink (mottled) “256” Cartons of 30 (3 x10) blisters 62332-103-30 15 mg light pink(mottled) “L257” Cartons of 30 (3 x10) blisters 62333-104-30 16.2 Storage Tablets Store at 20° to 25°C (68° to 77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Aripiprazole Orally Disintegrating Tablets, USP are indicated for the treatment of: •Schizophrenia [see Clinical Studies (14.1)] • Irritability Associated with Autistic Disorder [see Clinical Studies (14.4)] • Treatment of Tourette’s Disorder [see Clinical Studies (14.5)] Additional pediatric use information is approved for Otsuka America Pharmaceutical, Inc.’s ABILIFY® (aripiprazole) product. However, due to Otsuka America Pharmaceutical, Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. Aripiprazole is an atypical antipsychotic. The oral formulations are indicated for: • Schizophrenia (14.1) • Irritability Associated with Autistic Disorder (14.4) • Treatment of Tourette’s disorder (14.5)
Dosage and Administration
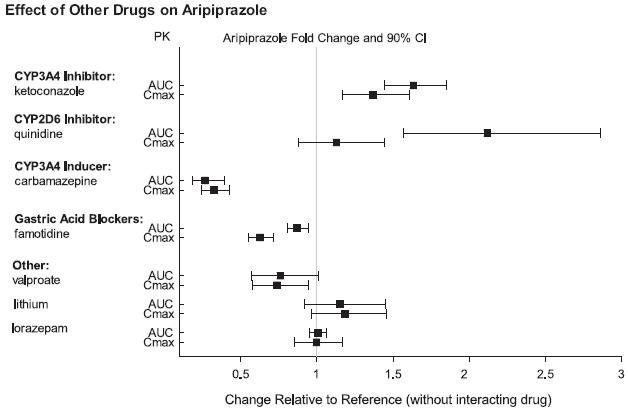
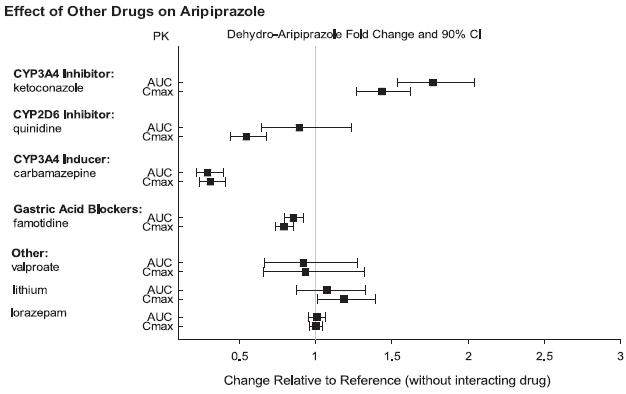
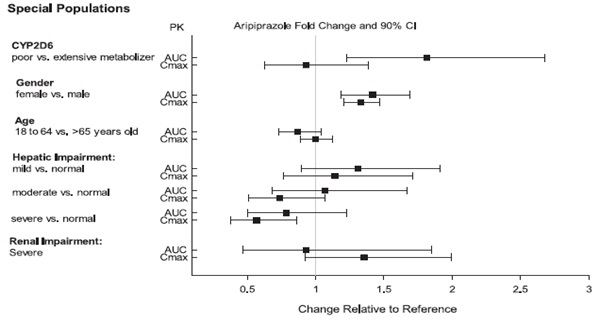
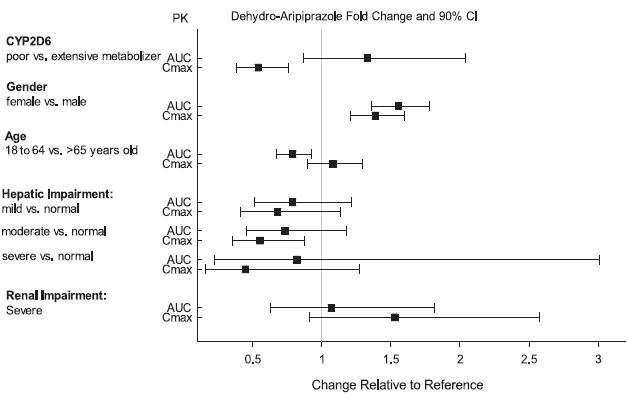
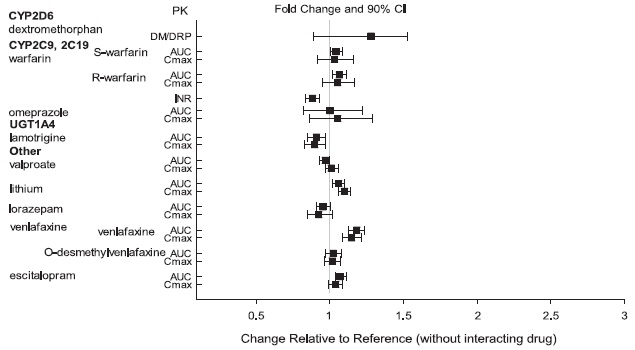
2 DOSAGE AND ADMINISTRATION Initial Dose Recommended Dose Maximum Dose Schizophrenia – adults (2.1) 10 to 15 mg /day 10 to 15 mg /day 30 mg /day Schizophrenia – adolescents (2.1) 2 mg /day 10 mg /day 30 mg /day Irritability associated with autistic disorder – pediatric patients (2.4) 2 mg/day 5 to 10 mg/day 15 mg/day Tourette’s disorder – (2.5) Patients < 50 kg 2 mg/day 5 mg/day 10 mg/day Patients ≥ 50 kg 2 mg/day 10 mg/day 20 mg/day •Oral formulations: Administer once daily without regard to meals (2) •Known CYP2D6 poor metabolizers: Half of the usual dose (2.7) 2.1 Schizophrenia Adults The recommended starting and target dose for aripiprazole is 10 or 15 mg/day administered on a once-a-day schedule without regard to meals. Aripiprazole has been systematically evaluated and shown to be effective in a dose range of 10 to 30 mg/day, when administered as the tablet formulation; however, doses higher than 10 or 15 mg/day were not more effective than 10 or 15 mg/day. Dosage increases should generally not be made before 2 weeks, the time needed to achieve steady-state [see Clinical Studies (14.1)] . Maintenance Treatment: Maintenance of efficacy in schizophrenia was demonstrated in a trial involving patients with schizophrenia who had been symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from those medications and randomized to either aripiprazole orally disintegrating tablets 15 mg/day or placebo, and observed for relapse [see Clinical Studies (14.1)] . Patients should be periodically reassessed to determine the continued need for maintenance treatment. Adolescents The recommended target dose of aripiprazole orally disintegrating tablets is 10 mg/day. Aripiprazole was studied in adolescent patients 13 to 17 years of age with schizophrenia at daily doses of 10 mg and 30 mg. The starting daily dose of the tablet formulation in these patients was 2 mg, which was titrated to 5 mg after 2 days and to the target dose of 10 mg after 2 additional days. Subsequent dose increases should be administered in 5 mg increments. The 30 mg/day dose was not shown to be more efficacious than the 10 mg/day dose. Aripiprazole orally disintegrating tablets can be administered without regard to meals [see Clinical Studies (14.1)] . Patients should be periodically reassessed to determine the continued need for maintenance treatment. Switching from Other Antipsychotics There are no systematically collected data to specifically address switching patients with schizophrenia from other antipsychotics to aripiprazole or concerning concomitant administration with other antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized. Additional pediatric use information is approved for Otsuka America Pharmaceutical, Inc.’s ABILIFY® (aripiprazole) product. However, due to Otsuka America Pharmaceutical, Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. 2.4 Irritability Associated with Autistic Disorder Pediatric Patients (6 to 17 years) The recommended dosage range for the treatment of pediatric patients with irritability associated with autistic disorder is 5 to 15 mg/day. Dosing should be initiated at 2 mg/day. The dose should be increased to 5 mg/day, with subsequent increases to 10 or 15 mg/day if needed. Dose adjustments of up to 5 mg/day should occur gradually, at intervals of no less than 1 week [see Clinical Studies (14.4)]. Patients should be periodically reassessed to determine the continued need for maintenance treatment. 2.5 Tourette’s Disorder Pediatric Patients (6 to 18 years) The recommended dosage range for Tourette’s Disorder is 5 to 20 mg/day. For patients weighing less than 50 kg, dosing should be initiated at 2 mg/day with a target dose of 5 mg/day after 2 days. The dose can be increased to 10 mg/day in patients who do not achieve optimal control of tics. Dosage adjustments should occur gradually at intervals of no less than 1 week. For patients weighing 50 kg or more, dosing should be initiated at 2 mg/day for 2 days, and then increased to 5 mg/day for 5 days, with a target dose of 10 mg/day on day 8. The dose can be increased up to 20 mg/day for patients who do not achieve optimal control of tics. Dosage adjustments should occur gradually in increments of 5 mg/day at intervals of no less than 1 week. [See Clinical Studies (14.5)]. Patients should be periodically reassessed to determine the continued need for maintenance treatment. 2.7 Dosage Adjustments for Cytochrome P450 Considerations Dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in patients taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers (see Table 2). When the coadministered drug is withdrawn from the combination therapy, aripiprazole orally disintegrating tablets dosage should then be adjusted to its original level. When the coadministered CYP3A4 inducer is withdrawn, aripiprazole orally disintegrating tablets dosage should be reduced to the original level over 1 to 2 weeks. Patients who may be receiving a combination of strong, moderate, and weak inhibitors of CYP3A4 and CYP2D6 (e.g., a strong CYP3A4 inhibitor and a moderate CYP2D6 inhibitor or a moderate CYP3A4 inhibitor with a moderate CYP2D6 inhibitor), the dosing may be reduced to one-quarter (25%) of the usual dose initially and then adjusted to achieve a favorable clinical response. Table 2: Dose Adjustments for Aripiprazole Orally Disintegrating Tablets in Patients who are known CYP2D6 Poor Metabolizers and Patients Taking Concomitant CYP2D6 Inhibitors, 3A4 Inhibitors, and/or CYP3A4 Inducers Factors Dosage Adjustments for A ripiprazole Orally Disintegrating Tablets Known CYP2D6 Poor Metabolizers Administer half of usual dose Known CYP2D6 Poor Metabolizers taking concomitant strong CYP3A4 inhibitors (e.g., itraconazole, clarithromycin) Administer a quarter of usual dose Strong CYP2D6 (e.g., quinidine, fluoxetine, paroxetine) or CYP3A4 inhibitors (e.g., itraconazole, clarithromycin) Administer half of usual dose Strong CYP2D6 and CYP3A4 inhibitors Administer a quarter of usual dose Strong CYP3A4 inducers (e.g., carbamazepine, rifampin) Double usual dose over 1 to 2 weeks 2.8 Dosing of Oral Solution The oral solution can be substituted for tablets on a mg-per-mg basis up to the 25 mg dose level. Patients receiving 30 mg tablets should receive 25 mg of the solution [see Clinical Pharmacology (12.3)]. 2.9 Dosing of Orally Disintegrating Tablets The dosing for aripiprazole orally disintegrating tablets is the same as for the oral tablets [see Dosage and Administration (2.1 and 2.4)].
