Drug Catalog - Product Detail
ARIPIPRAZOLE TABLETS 2MG 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 43598-0554-30 | DR.REDDY'S LABORATORIES, INC. | 30 | 2MG | TABLET |
PACKAGE FILES










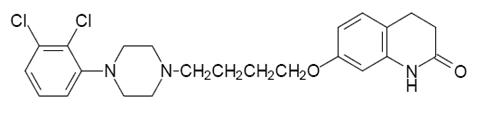
Generic Name
ARIPIPRAZOLE
Substance Name
ARIPIPRAZOLE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA206383
Description
11 DESCRIPTION Aripiprazole is a psychotropic drug that is available as tablets. Aripiprazole USP is 7-[4-[4-(2,3-dichlorophenyl)-1piperazinyl]butoxy]-3,4-dihydrocarbostyril. The empirical formula is C 23 H 27 Cl 2 N 3 O 2 and its molecular weight is 448.39. The chemical structure is: Aripiprazole Tablets, USP are available in 2 mg, 5 mg, 10 mg, 15 mg, 20 mg, and 30 mg strengths. Inactive ingredients include corn starch, colloidal silicon dioxide, isopropyl alcohol, lactose monohydrate, microcrystalline cellulose, magnesium stearate, povidone. Colorants include ferric oxide (yellow or red) and FD&C Blue No. 2 Aluminum Lake. FDA approved dissolution test specifications differ from USP. structural formula
How Supplied
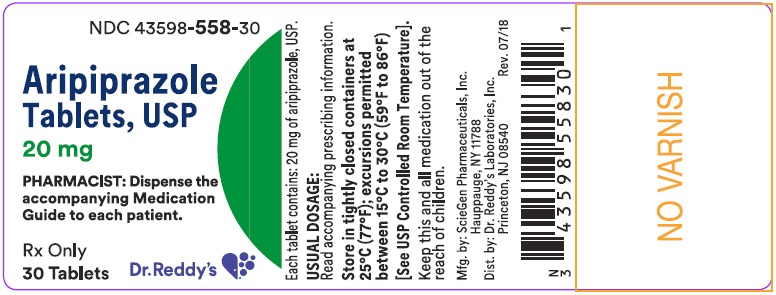
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Aripiprazole Tablets, USP have markings on one side and are available in the strengths and packages listed in Table 32. Table 32: Aripiprazole Tablets, USP Presentations Tablet Strength Tablet Color/ Shape Tablet Markings Pack Size NDC Code 2 mg green modified rectangle “SG 324” Bottle of 30 Bottle of 500 43598-554-30 43598-554-05 5 mg blue modified rectangle “SG 325” Bottle of 30 Bottle of 500 43598-555-30 43598-555-05 10 mg pink modified rectangle “SG 326” Bottle of 30 Bottle of 500 43598-556-30 43598-556-05 15 mg pink round “SG 327” Bottle of 30 Bottle of 500 43598-557-30 43598-557-05 20 mg pink round “SG 328” Bottle of 30 Bottle of 500 43598-558-30 43598-558-05 30 mg pink round “SG 329” Bottle of 30 Bottle of 500 43598-559-30 43598-559-05 16.2 Storage Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Aripiprazole tablets are indicated for the treatment of: • Schizophrenia [ see CLINICAL STUDIES ( 14.1 ) ] Additional pediatric use information is approved for Otsuka America Pharmaceutical, Inc.’s ABILIFY ® (aripiprazole) product. However, due to Otsuka America Pharmaceutical, Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. Aripiprazole is an atypical antipsychotic. The oral formulation is indicated for: • Schizophrenia ( 14.1 )
Dosage and Administration
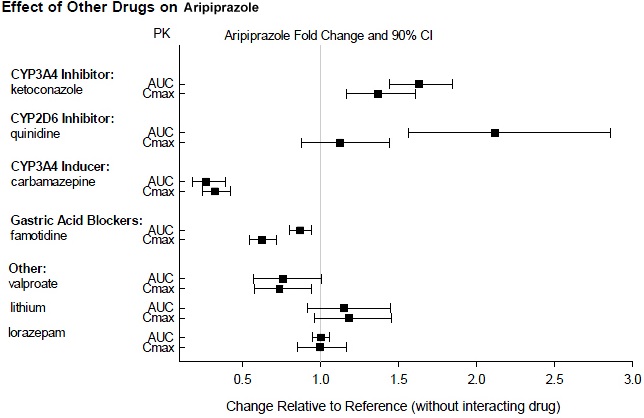
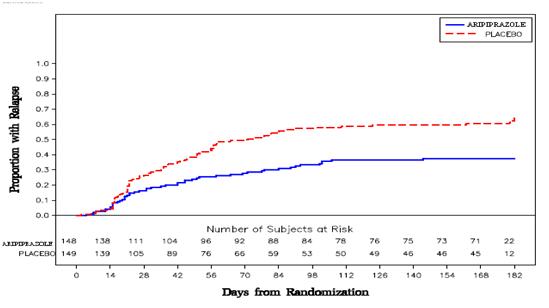
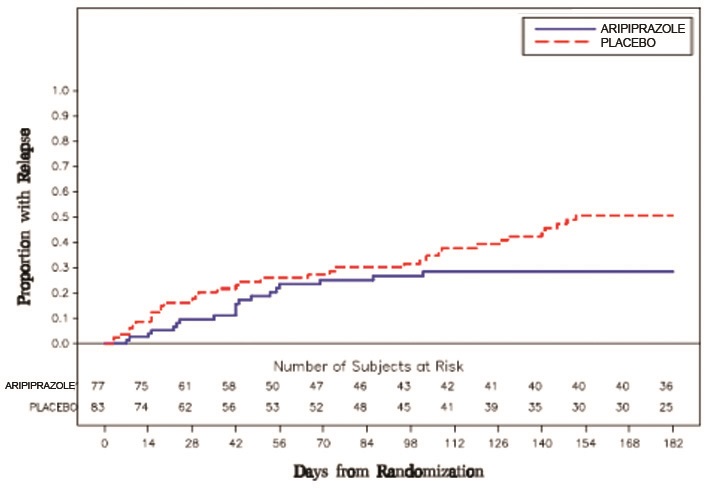
2 DOSAGE AND ADMINISTRATION Initial Dose Recommended Dose Maximum Dose Schizophrenia – adults ( 2.1 ) 10 mg /day to 15 mg /day 10 mg /day to 15 mg /day 30 mg /day Schizophrenia – adolescents ( 2.1 ) 2 mg /day 10 mg /day 30 mg /day 2.1 Schizophrenia Adults The recommended starting and target dose for aripiprazole is 10 mg/day or 15 mg/day administered on a once-a-day schedule without regard to meals. Aripiprazole has been systematically evaluated and shown to be effective in a dose range of 10 mg/day to 30 mg/day, when administered as the tablet formulation; however, doses higher than 10 mg/day or 15 mg/day were not more effective than 10 mg/day or 15 mg/day. Dosage increases should generally not be made before 2 weeks, the time needed to achieve steady-state [ see CLINICAL STUDIES ( 14.1 ) ]. Maintenance Treatment: Maintenance of efficacy in schizophrenia was demonstrated in a trial involving patients with schizophrenia who had been symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from those medications and randomized to either aripiprazole 15 mg/day or placebo, and observed for relapse [ see CLINICAL STUDIES ( 14.1 ) ]. Patients should be periodically reassessed to determine the continued need for maintenance treatment. Adolescents The recommended target dose of aripiprazole is 10 mg/day. Aripiprazole was studied in adolescent patients 13 to 17 years of age with schizophrenia at daily doses of 10 mg and 30 mg. The starting daily dose of the tablet formulation in these patients was 2 mg, which was titrated to 5 mg after 2 days and to the target dose of 10 mg after 2 additional days. Subsequent dose increases should be administered in 5 mg increments. The 30 mg/day dose was not shown to be more efficacious than the 10 mg/day dose. Aripiprazole can be administered without regard to meals [ see CLINICAL STUDIES ( 14.1 ) ]. Patients should be periodically reassessed to determine the need for maintenance treatment. Switching from Other Antipsychotics There are no systematically collected data to specifically address switching patients with schizophrenia from other antipsychotics to aripiprazole or concerning concomitant administration with other antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized. Additional pediatric use information is approved for Otsuka America Pharmaceutical, Inc.’s ABILIFY ® (aripiprazole) product. However, due to Otsuka America Pharmaceutical, Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. 2.7 Dosage Adjustments for Cytochrome P450 Considerations Dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in patients taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers (see Table 2). When the coadministered drug is withdrawn from the combination therapy, aripiprazole dosage should then be adjusted to its original level. When the coadministered CYP3A4 inducer is withdrawn, aripiprazole dosage should be reduced to the original level over 1 to 2 weeks. Patients who may be receiving a combination of strong, moderate, and weak inhibitors of CYP3A4 and CYP2D6 (e.g., a strong CYP3A4 inhibitor and a moderate CYP2D6 inhibitor or a moderate CYP3A4 inhibitor with a moderate CYP2D6 inhibitor), the dosing may be reduced to one-quarter (25%) of the usual dose initially and then adjusted to achieve a favorable clinical response. Table 2: Dose Adjustments for aripiprazole in Patients who are known CYP2D6 Poor Metabolizers and Patients Taking Concomitant CYP2D6 Inhibitors, 3A4 Inhibitors, and/or CYP3A4 Inducers Factors Dosage Adjustments for Aripiprazole Tablets Known CYP2D6 Poor Metabolizers Administer half of usual dose Known CYP2D6 Poor Metabolizers taking concomitant strong CYP3A4 inhibitors (e.g., itraconazole, clarithromycin) Administer a quarter of usual dose Strong CYP2D6 (e.g., quinidine, fluoxetine, paroxetine) or CYP3A4 inhibitors (e.g., itraconazole, clarithromycin) Administer half of usual dose Strong CYP2D6 and CYP3A4 inhibitors Administer a quarter of usual dose Strong CYP3A4 inducers (e.g., carbamazepine, rifampin) Double usual dose over 1 to 2 weeks 2.8 Dosing of Oral Solution The oral solution can be substituted for tablets on a mg-per-mg basis up to the 25 mg dose level. Patients receiving 30 mg tablets should receive 25 mg of the solution [see CLINICAL PHARMACOLOGY (12.3) ].
