Drug Catalog - Product Detail
Aspirin-Dipyridamole Cap ER 12HR 25-200 MG 60 EA UoU
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0405-60 | GLENMARK PHARMACEUTICALS | 60 | 25-200MG | NA |
PACKAGE FILES


Generic Name
ASPRIN AND EXTENDED-RELEASE DIPYRIDAMOLE
Substance Name
ASPIRIN
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA210318
Description
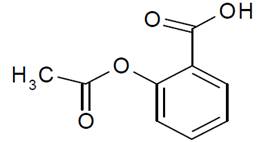
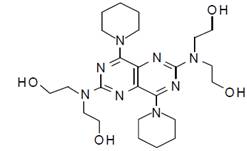
11 DESCRIPTION Aspirin and Extended-Release Dipyridamole Capsules are a combination of aspirin and dipyridamole, antiplatelet agents, intended for oral administration. Each hard gelatin capsule contains 200 mg dipyridamole, USP in an extended-release form and 25 mg aspirin, USP as an immediate-release sugar-coated tablet. In addition, each capsule contains the following inactive ingredients: acacia milled powder, eudragit S-100, glyceryl behenate, hydroxy propyl methyl cellulose phthalate HP-55, hypromellose, hypromellose E3LV, hypromellose E50LV, lactose monohydrate, microcrystalline cellulose, micronized talc, povidone K 30, silicon dioxide, talc, tartatic acid pellets and triacetin. Each capsule shell contains FD & C blue 1, FD & C red 3, FD & C red 40, FD & C yellow 6, gelatin, iron oxide yellow, sodium lauryl sulphate and titanium dioxide. The imprinting ink contains black iron oxide, potassium hydroxide, and shellac. Dipyridamole Dipyridamole is an antiplatelet agent chemically described as 2,2',2'',2'''-[(4,8-Dipiperidinopyrimido[5,4- d ]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula: Molecular formula: C 24 H 40 N 8 O 4 Mol. Wt. 504.63 g/mol Dipyridamole is an odorless yellow crystalline substance, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and is practically insoluble in water. Aspirin The antiplatelet agent aspirin (acetylsalicylic acid) is chemically known as benzoic acid, 2- (acetyloxy)-, and has the following structural formula: Molecular formula: C 9 H 8 O 4 Mol . Wt. 180.16 g/mol Aspirin has white crystals, commonly tubular or needle-like, or white, crystalline powder. When exposed to moisture, aspirin hydrolyzes into salicylic and acetic acids, and gives off a vinegary odor. It is slightly soluble in water; freely soluble in alcohol; soluble in chloroform and in ether; sparingly soluble in absolute ether. dipyridamole-structure.jpg asprin-structure.jpg
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Aspirin and Extended-Release Dipyridamole Capsules, 25 mg/200 mg are available as size ‘0el’ empty hard gelatin capsules with a reddish brown opaque cap and a cream opaque body, imprinted with the Glenmark logo ‘G’ on the cap and ‘405’ on the body with black ink. The capsules are filled with light yellow to yellow colored dipyridamole pellets in extended-release form and a white to off-white aspirin tablet in immediate-release form. Aspirin and Extended-Release Dipyridamole Capsules, 25 mg/200 mg are supplied in unit-of-use bottles of 30 capsules (NDC 68462-405-30) and unit-of-use bottles of 60 capsules (NDC 68462-405-60). Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from excessive moisture.
Indications & Usage
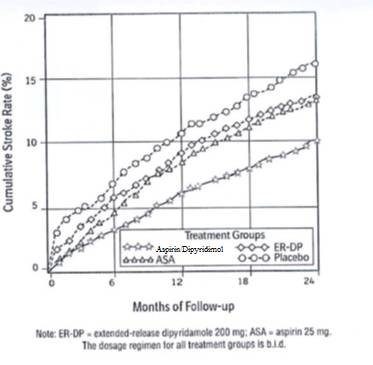
1 INDICATIONS AND USAGE Aspirin and extended-release dipyridamole capsules are indicated to reduce the risk of stroke in patients who have had transient ischemia of the brain or completed ischemic stroke due to thrombosis. • Aspirin and extended-release dipyridamole capsules are a combination of aspirin and dipyridamole, antiplatelet agents, indicated to reduce the risk of stroke in patients who have had transient ischemia of the brain or completed ischemic stroke due to thrombosis ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Aspirin and extended-release dipyridamole capsules are not interchangeable with the individual components of aspirin and dipyridamole tablets. The recommended dose of aspirin and extended-release dipyridamole capsules is one capsule given orally twice daily, one in the morning and one in the evening. Swallow capsules whole without chewing. Aspirin and extended-release dipyridamole capsules can be administered with or without food. • One capsule twice daily (morning and evening) with or without food ( 2 ) • In case of intolerable headaches during initial treatment, switch to one capsule at bedtime and low-dose aspirin in the morning; resume BID dosing within one week ( 2.1 ) • Do not chew capsule ( 2 ) • Not interchangeable with the individual components of aspirin and dipyridamole tablets ( 2 ) • Dispense in this unit-of-use container ( 16 ) 2.1 Alternative Regimen in Case of Intolerable Headaches In the event of intolerable headaches during initial treatment, switch to one capsule at bedtime and low-dose aspirin in the morning. Because there are no outcome data with this regimen and headaches become less of a problem as treatment continues, patients should return to the usual regimen as soon as possible, usually within one week.
