Drug Catalog - Product Detail
ATORVASTATIN CALCIUM 10MG TB 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00781-5381-92 | SANDOZ | 90 | 10MG | TABLET |
PACKAGE FILES






Generic Name
ATORVASTATIN CALCIUM
Substance Name
ATORVASTATIN CALCIUM TRIHYDRATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077575
Description
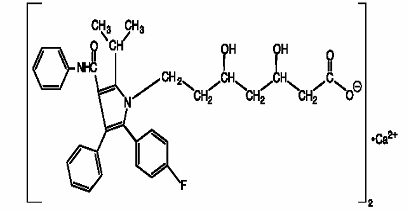
11 DESCRIPTION Atorvastatin calcium tablets are a synthetic lipid-lowering agent. Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. This enzyme catalyzes the conversion of HMG-CoA to mevalonate, an early and rate-limiting step in cholesterol biosynthesis. Atorvastatin calcium is [R-(R*, R*)]-2-(4-fluorophenyl)-ß, δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid, calcium salt (2:1). The molecular formula of atorvastatin calcium is (C 33 H 34 FN 2 O 5 ) 2 Ca and its molecular weight is 1155.38. Its structural formula is: Atorvastatin calcium is a white to off-white powder that is insoluble in aqueous solutions of pH 4 and below. Atorvastatin calcium is very slightly soluble in distilled water, pH 7.4 phosphate buffer, and acetonitrile; slightly soluble in ethanol; and freely soluble in methanol. Atorvastatin calcium tablets for oral administration contain 10, 20, 40, or 80 mg atorvastatin and the following inactive ingredients: carboxymethylcellulose sodium, ferric oxide yellow, glycerin, magnesium stearate, pregelatinized starch, silicified microcrystalline cellulose, sodium lauryl sulfate, sodium starch glycolate, talc, tromethamine. Chemical Structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Atorvastatin calcium tablets are supplied as follows: 10 mg, light yellow, dappled, glossy, round biconvex film-coated tablets, debossed with “HLA 10” on one side. Bottles contain desiccant. NDC 0781-5381-31, bottle of 30 tablets NDC 0781-5381-92, bottle of 90 tablets NDC 0781-5381-01, bottle of 100 tablets NDC 0781-5381-10, bottle of 1000 tablets NDC 0781-5381-34, bottle of 3000 tablets 20 mg, light yellow, dappled, glossy, round biconvex film-coated tablets, debossed with “HLA 20” on one side. Bottles contain desiccant. NDC 0781-5382-31, bottle of 30 tablets NDC 0781-5382-92, bottle of 90 tablets NDC 0781-5382-01, bottle of 100 tablets NDC 0781-5382-10, bottle of 1000 tablets NDC 0781-5382-34, bottle of 3000 tablets 40 mg, light yellow, dappled, glossy, round biconvex film-coated tablets, debossed with “HLA 40” on one side. Bottles contain desiccant. NDC 0781-5384-31, bottle of 30 tablets NDC 0781-5384-92, bottle of 90 tablets NDC 0781-5384-01, bottle of 100 tablets NDC 0781-5384-10, bottle of 1000 tablets NDC 0781-5384-22, bottle of 2000 tablets 80 mg, light yellow, dappled, glossy, oval biconvex film-coated tablets, debossed with “HLA 80” on one side. Bottles contain desiccant. NDC 0781-5388-31, bottle of 30 tablets NDC 0781-5388-92, bottle of 90 tablets NDC 0781-5388-01, bottle of 100 tablets NDC 0781-5388-10, bottle of 1000 tablets Storage Store at 20º to 25ºC (68º to 77ºF) [see USP Controlled Room Temperature]. Protect from moisture. Dispense in a tight, light-resistant container.
Indications & Usage
1 INDICATIONS AND USAGE Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Drug therapy is recommended as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other nonpharmacologic measures alone has been inadequate. In patients with CHD or multiple risk factors for CHD, atorvastatin calcium tablets can be started simultaneously with diet. Atorvastatin calcium tablets are an inhibitor of HMG-CoA reductase (statin) indicated as an adjunct therapy to diet to: • Reduce the risk of MI, stroke, revascularization procedures, and angina in patients without CHD, but with multiple risk factors ( 1.1 ). • Reduce the risk of MI and stroke in patients with type 2 diabetes without CHD, but with multiple risk factors ( 1.1 ). • Reduce the risk of non-fatal MI, fatal and non-fatal stroke, revascularization procedures, hospitalization for CHF, and angina in patients with CHD ( 1.1 ). • Reduce elevated total-C, LDL-C, apo B, and TG levels and increase HDL-C in adult patients with primary hyperlipidemia (heterozygous familial and nonfamilial) and mixed dyslipidemia ( 1.2 ). • Reduce elevated TG in patients with hypertriglyceridemia and primary dysbetalipoproteinemia ( 1.2 ). • Reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia (HoFH) ( 1.2 ). • Reduce elevated total-C, LDL-C, and apo B levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia after failing an adequate trial of diet therapy ( 1.2 ). Limitations of Use Atorvastatin calcium tablets have not been studied in Fredrickson Types I and V dyslipidemias. 1.1 Prevention of Cardiovascular Disease In adult patients without clinically evident coronary heart disease, but with multiple risk factors for coronary heart disease such as age, smoking, hypertension, low HDL-C, or a family history of early coronary heart disease, atorvastatin calcium tablets are indicated to: • Reduce the risk of myocardial infarction • Reduce the risk of stroke • Reduce the risk for revascularization procedures and angina In patients with type 2 diabetes, and without clinically evident coronary heart disease, but with multiple risk factors for coronary heart disease such as retinopathy, albuminuria, smoking, or hypertension, atorvastatin calcium tablets are indicated to: • Reduce the risk of myocardial infarction • Reduce the risk of stroke In patients with clinically evident coronary heart disease, atorvastatin calcium tablets are indicated to: • Reduce the risk of non-fatal myocardial infarction • Reduce the risk of fatal and non-fatal stroke • Reduce the risk for revascularization procedures • Reduce the risk of hospitalization for CHF • Reduce the risk of angina 1.2 Hyperlipidemia Atorvastatin calcium tablets are indicated: • As an adjunct to diet to reduce elevated total-C, LDL-C, apo B, and TG levels and to increase HDL-C in patients with primary hypercholesterolemia (heterozygous familial and nonfamilial) and mixed dyslipidemia (Fredrickson Types IIa and IIb); • As an adjunct to diet for the treatment of patients with elevated serum TG levels (Fredrickson Type IV); • For the treatment of patients with primary dysbetalipoproteinemia (Fredrickson Type III) who do not respond adequately to diet; • To reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable; • As an adjunct to diet to reduce total-C, LDL-C, and apo B levels in boys and postmenarchal girls, 10 to 17 years of age, with heterozygous familial hypercholesterolemia if after an adequate trial of diet therapy the following findings are present: a. LDL-C remains ≥ 190 mg/dL or b. LDL-C remains ≥ 160 mg/dL and: ▪ there is a positive family history of premature cardiovascular disease or ▪ two or more other CVD risk factors are present in the pediatric patient 1.3 Limitations of Use Atorvastatin calcium tablets have not been studied in conditions where the major lipoprotein abnormality is elevation of chylomicrons (Fredrickson Types I and V).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Dose range: 10 to 80 mg once daily ( 2.1 ). Recommended start dose: 10 or 20 mg once daily ( 2.1 ). Patients requiring large LDL-C reduction (>45%) may start at 40 mg once daily ( 2.1 ). Pediatric starting dose: 10 mg once daily; maximum recommended dose: 20 mg once daily ( 2.2 ). 2.1 Hyperlipidemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia (Fredrickson Types IIa and IIb) The recommended starting dose of atorvastatin calcium tablets is 10 or 20 mg once daily. Patients who require a large reduction in LDL-C (more than 45%) may be started at 40 mg once daily. The dosage range of atorvastatin calcium tablets is 10 to 80 mg once daily. Atorvastatin calcium tablets can be administered as a single dose at any time of the day, with or without food. The starting dose and maintenance doses of atorvastatin calcium tablets should be individualized according to patient characteristics such as goal of therapy and response (see current NCEP Guidelines ). After initiation and/or upon titration of atorvastatin calcium tablets, lipid levels should be analyzed within 2 to 4 weeks and dosage adjusted accordingly. 2.2 Heterozygous Familial Hypercholesterolemia in Pediatric Patients (10 to 17 years of age) The recommended starting dose of atorvastatin calcium tablets is 10 mg/day; the maximum recommended dose is 20 mg/day (doses greater than 20 mg have not been studied in this patient population). Doses should be individualized according to the recommended goal of therapy [see current NCEP Pediatric Panel Guidelines , CLINICAL PHARMACOLOGY (12) , and INDICATIONS AND USAGE (1.2 ) ]. Adjustments should be made at intervals of 4 weeks or more. 2.3 Homozygous Familial Hypercholesterolemia The dosage of atorvastatin calcium tablets in patients with homozygous FH is 10 to 80 mg daily. Atorvastatin calcium tablets should be used as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) in these patients or if such treatments are unavailable. 2.4 Concomitant Lipid-Lowering Therapy Atorvastatin calcium tablets may be used with bile acid resins. The combination of HMG-CoA reductase inhibitors (statins) and fibrates should generally be used with caution [see WARNINGS AND PRECAUTIONS, Skeletal Muscle (5.1) , DRUG INTERACTIONS (7) ]. 2.5 Dosage in Patients With Renal Impairment Renal disease does not affect the plasma concentrations nor LDL-C reduction of atorvastatin calcium tablets; thus, dosage adjustment in patients with renal dysfunction is not necessary [see WARNINGS AND PRECAUTIONS, Skeletal Muscle (5.1) , CLINICAL PHARMACOLOGY, Pharmacokinetics (12.3) ]. 2.6 Dosage in Patients Taking Cyclosporine, Clarithromycin, Itraconazole, or Certain Protease Inhibitors In patients taking cyclosporine or the HIV protease inhibitors (tipranavir plus ritonavir) or the hepatitis C protease inhibitor (telaprevir), therapy with atorvastatin calcium tablets should be avoided. In patients with HIV taking lopinavir plus ritonavir, caution should be used when prescribing atorvastatin calcium tablets and the lowest dose necessary employed. In patients taking clarithromycin, itraconazole, or in patients with HIV taking a combination of saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, or fosamprenavir plus ritonavir, therapy with atorvastatin calcium tablets should be limited to 20 mg, and appropriate clinical assessment is recommended to ensure that the lowest dose necessary of atorvastatin calcium tablets are employed. In patients taking the HIV protease inhibitor nelfinavir or the hepatitis C protease inhibitor boceprevir, therapy with atorvastatin calcium tablets should be limited to 40 mg, and appropriate clinical assessment is recommended to ensure that the lowest dose necessary of atorvastatin calcium tablets is employed [see WARNINGS AND PRECAUTIONS, Skeletal Muscle (5.1) , DRUG INTERACTIONS (7) ].
