Drug Catalog - Product Detail
ATORVASTATIN CALCIUM TB 10MG 10X10 UD
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00904-6290-61 | MAJOR PHARMACEUTICALS | 100 | 10MG | TABLET |
PACKAGE FILES




Generic Name
ATORVASTATIN CALCIUM
Substance Name
ATORVASTATIN CALCIUM PROPYLENE GLYCOL SOLVATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090548
Description
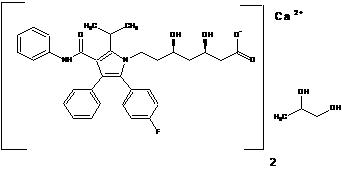
11 DESCRIPTION Atorvastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. The drug substance used in atorvastatin calcium tablets, USP is atorvastatin calcium in the form of propylene glycol solvate. The chemical name for atorvastatin calcium propylene glycol solvate is calcium bis((3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoate) propylene glycol solvate. The empirical formula of atorvastatin calcium propylene glycol solvate is C 66 H 68 CaF 2 N 4 O 10 * C 3 H 8 O 2 and its molecular weight is 1231.46 g/mol. Its structural formula is: Atorvastatin calcium is a white to off-white solid that is insoluble in aqueous solutions of pH 4 and below. Atorvastatin calcium is slightly soluble in distilled water, pH 7.4 phosphate buffer, and acetonitrile; slightly soluble in ethanol; and freely soluble in methanol. Atorvastatin calcium tablets, USP for oral administration contain atorvastatin 10, 20, 40, or 80 mg (equivalent to 11, 22, 44 or 88 mg atorvastatin calcium) and the following inactive ingredients: calcium acetate, colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, hypromellose, magnesium stearate (vegetable source), microcrystalline cellulose, polyethylene glycol, sodium carbonate, and titanium dioxide. chemical-structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Atorvastatin calcium tablets, USP are supplied as white to off-white, oval, biconvex film-coated tablets of atorvastatin calcium containing 10, 20, 40 and 80 mg atorvastatin. 10 mg tablets Atorvastatin calcium tablets, USP 10 mg, are available for oral administration as white to off-white, oval, biconvex film-coated tablets, engraved “APO” on one side, “A10” on the other side. Carton of 50 tablets (10 tablets each blister pack x 5) NDC 0904-6290-06 Carton of 100 tablets (10 tablets each blister pack x 10) NDC 0904-6290-61 20 mg tablets Atorvastatin calcium tablets, USP 20 mg, are available for oral administration as white to off-white, oval, biconvex film-coated tablets, engraved “APO” on one side, “ATV20” on the other side. Carton of 50 tablets (10 tablets each blister pack x 5) NDC 0904-6291-06 Carton of 100 tablets (10 tablets each blister pack x 10) NDC 0904-6291-61 Storage Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Dispense in a tight container [see USP].
Indications & Usage
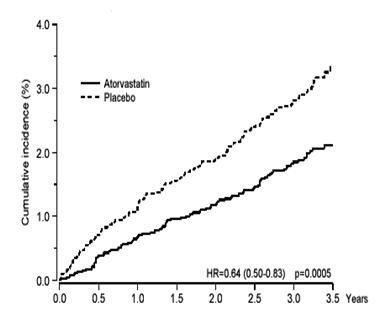
1 INDICATIONS AND USAGE Atorvastatin Calcium Tablets are indicated: • To reduce the risk of: o Myocardial infarction (MI), stroke, revascularization procedures, and angina in adults with multiple risk factors for coronary heart disease (CHD) but without clinically evident CHD o MI and stroke in adults with type 2 diabetes mellitus with multiple risk factors for CHD but without clinically evident CHD o Non-fatal MI, fatal and non-fatal stroke, revascularization procedures, hospitalization for congestive heart failure, and angina in adults with clinically evident CHD • As an adjunct to diet to reduce low-density lipoprotein cholesterol (LDL-C) in: o Adults with primary hyperlipidemia. o Adults and pediatric patients aged 10 years and older with heterozygous familial hypercholesterolemia (HeFH). • As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 10 years and older with homozygous familial hypercholesterolemia (HoFH). • As an adjunct to diet for the treatment of adults with: o Primary dysbetalipoproteinemia o Hypertriglyceridemia Atorvastatin calcium tablets are an HMG-CoA reductase inhibitor (statin) indicated ( 1 ): • To reduce the risk of: o Myocardial infarction (MI), stroke, revascularization procedures, and angina in adults with multiple risk factors for coronary heart disease (CHD) but without clinically evident CHD. o MI and stroke in adults with type 2 diabetes mellitus with multiple risk factors for CHD but without clinically evident CHD. o Non-fatal MI, fatal and non-fatal stroke, revascularization procedures, hospitalization for congestive heart failure, and angina in adults with clinically evident CHD. • As an adjunct to diet to reduce low-density lipoprotein (LDL-C) in: o Adults with primary hyperlipidemia. o Adults and pediatric patients aged 10 years and older with heterozygous familial hypercholesterolemia (HeFH). • As an adjunct to other LDL-C-lowering therapies to reduce LDL-C in adults and pediatric patients aged 10 years and older with homozygous familial hypercholesterolemia. • As an adjunct to diet for the treatment of adults with: o Primary dysbetaliproteinemia. o Hypertriglyceridemia.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Take orally once daily with or without food ( 2.1 ). • Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating atorvastatin calcium, and adjust dosage if necessary ( 2.1 ). • Adults ( 2.2 ): o Recommended starting dosage is 10 or 20 mg once daily; dosage range is 10 mg to 80 mg once daily. o Patients requiring LDL-C reduction >45% may start at 40 mg once daily. • Pediatric Patients Aged 10 Years of Age and Older with HeFH: Recommended starting dosage is 10 mg once daily; dosage range is 10 to 20 mg once daily ( 2.3 ). • Pediatric Patients Aged 10 Years of Age and Older with HoFH: Recommended starting dosage is 10 to 20 mg once daily; dosage range is 10 to 80 mg once daily ( 2.4 ). • See full prescribing information for atorvastatin calcium tablets dosage modifications due to drug interactions ( 2.5 ). 2.1 Important Dosage Information • Take Atorvastatin calcium tablet orally once daily at any time of the day, with or without food. • Assess LDL-C when clinically appropriate, as early as 4 weeks after initiating atorvastatin calcium tablets, and adjust the dosage if necessary. 2.2 Recommended Dosage in Adult Patients The recommended starting dosage of atorvastatin calcium is 10 mg to 20 mg once daily. The dosage range is 10 mg to 80 mg once daily. Patients who require reduction in LDL-C greater than 45% may be started at 40 mg once daily. 2.3 Recommended Dosage in Pediatric Patients 10 Years of Age and Older with HeFH The recommended starting dosage of atorvastatin calcium is 10 mg once daily. The dosage range is 10 mg to 20 mg once daily. 2.4 Recommended Dosage in Pediatric Patients 10 Years of Age and Older with HoFH The recommended starting dosage of atorvastatin calcium is 10 mg to 20 mg once daily. The dosage range is 10 mg to 80 mg once daily. 2.5 Dosage Modifications Due to Drug Interactions Concomitant use of atorvastatin with the following drugs requires dosage modification of atorvastatin [see Warnings and Precautions ( 5.1 ) and Drug Interactions ( 7.1 )] . Anti-Viral Medications • In patients taking saquinavir plus ritonavir, darunavir plus ritonavir, fosamprenavir, fosamprenavir plus ritonavir, elbasvir plus grazoprevir or letermovir, do not exceed atorvastatin calcium 20 mg once daily. • In patients taking nelfinavir, do not exceed atorvastatin calcium 40 mg once daily. Select Azole Antifungals or Macrolide Antibiotics • In patients taking clarithromycin or itraconazole, do not exceed atorvastatin calcium 20 mg once daily. For additional recommendations regarding concomitant use of atorvastatin calcium with other anti-viral medications, azole antifungals or macrolide antibiotics, see Drug Interactions ( 7.1 ).
