Drug Catalog - Product Detail
AZELASTINE HCL NASAL 137MCG SPR 30ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 47335-0779-91 | SUN PHARMACEUTICALS | 30 | 0.1% | SOLUTION |
PACKAGE FILES

Generic Name
AZELASTINE HYDROCHLORIDE
Substance Name
AZELASTINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
NASAL
Application Number
ANDA090423
Description
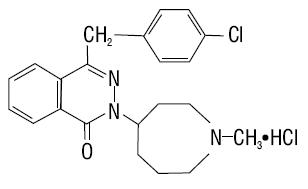
11 DESCRIPTION Azelastine Hydrochloride Nasal Solution (Nasal Spray), 0.1% (137 mcg per spray), is an antihistamine formulated as a metered-spray solution for intranasal administration. Azelastine hydrochloride occurs as a white, almost odorless, crystalline powder with a bitter taste. It has a molecular weight of 418.37. It is sparingly soluble in water, methanol, and propylene glycol and slightly soluble in ethanol, octanol, and glycerine. It has a melting point of about 225°C and the pH of a saturated solution is between 5 and 5.4. Its chemical name is (±)-1-(2H)-phthalazinone,4-[(4-chlorophenyl) methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride. Its molecular formula is C 22 H 24 ClN 3 O•HCl with the following chemical structure: Azelastine hydrochloride nasal solution contains 0.1% azelastine hydrochloride in an aqueous solution at pH 6.8 ± 0.3. It also contains benzalkonium chloride 50% solution (250 mcg/mL), edetate disodium, hypromellose, citric acid anhydrous, dibasic sodium phosphate, sodium chloride, and water for injection. After priming [ see Dosage and Administration (2.3) ], each metered spray delivers a 0.137 mL mean volume containing 137 mcg of azelastine hydrochloride (equivalent to 125 mcg of azelastine base). The bottle can deliver 200 metered sprays. chemicalstructure
How Supplied
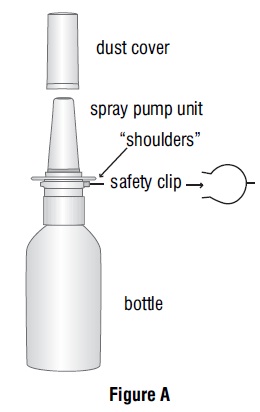
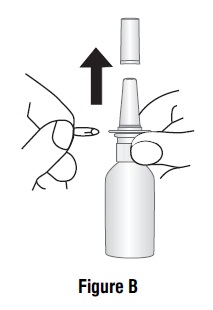
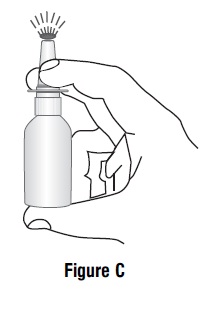
16 HOW SUPPLIED/STORAGE AND HANDLING Azelastine hydrochloride nasal solution (Nasal Spray), 0.1% (137 mcg per spray) is supplied as a 30 mL package (NDC 47335-779-91) delivering 200 metered sprays in a high-density polyethylene (HDPE) bottle fitted with a metered-dose spray pump unit. The spray pump unit consists of a nasal spray pump fitted with a blue safety clip and a blue plastic dust cover. The net content of the bottle is 30 mL (net weight 30 gm of solution). Each bottle contains 30 mg (1 mg/mL) of azelastine hydrochloride. After priming [ see Dosage and Administration ( 2.3 ) ], each spray delivers a fine mist containing a mean volume of 0.137 mL solution containing 137 mcg of azelastine hydrochloride. The correct amount of medication in each spray cannot be assured before the initial priming and after 200 sprays have been used, even though the bottle is not completely empty. The bottle should be discarded after 200 sprays have been used. Azelastine hydrochloride nasal solution (nasal spray) should not be used after the expiration date "Exp" printed on the medicine label and carton. Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature]. Protect from freezing.
Indications & Usage
1 INDICATIONS AND USAGE Azelastine hydrochloride nasal solution (nasal spray), 0.1% (137 mcg per spray) is indicated for the treatment of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 5 years and older, and for the treatment of the symptoms of vasomotor rhinitis in adults and adolescent patients 12 years and older. Azelastine hydrochloride nasal solution (nasal spray) is an H 1 -receptor antagonist indicated for the treatment of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 5 years and older and for the treatment of the symptoms of vasomotor rhinitis in adults and adolescent patients 12 years and older. ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION For intranasal use only ( 2.3 ) Seasonal allergic rhinitis: Pediatric patients 5 to 11 years of age: 1 spray per nostril twice daily ( 2.1 ) Adults and adolescents 12 years of age and older: 1 or 2 sprays per nostril twice daily ( 2.1 ) Vasomotor rhinitis: 2 sprays per nostril twice daily in adults and adolescents 12 years of age and older ( 2.2 ) Prime azelastine hydrochloride nasal solution before initial use and when it has not been used for 3 or more days ( 2.3 ) 2.1 Seasonal Allergic Rhinitis The recommended dosage of azelastine hydrochloride nasal solution in adults and adolescent patients 12 years and older with seasonal allergic rhinitis is one or two sprays per nostril twice daily. The recommended dosage of azelastine hydrochloride nasal solution in pediatric patients 5 years to 11 years of age is one spray per nostril twice daily. 2.2 Vasomotor Rhinitis The recommended dosage of azelastine hydrochloride nasal solution in adults and adolescent patients 12 years and older with vasomotor rhinitis is two sprays per nostril twice daily. 2.3 Important Administration Instructions Administer azelastine hydrochloride nasal solution by the intranasal route only. Priming: Prime azelastine hydrochloride nasal solution before initial use by releasing 4 sprays or until a fine mist appears. When azelastine hydrochloride nasal solution has not been used for 3 or more days, reprime with 2 sprays or until a fine mist appears. Avoid spraying azelastine hydrochloride nasal solution into the eyes.
