Drug Catalog - Product Detail
AZELASTINE HCL NASAL SPRAY 137MCG 30ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0676-84 | AMNEAL PHARMACEUTICALS | 30 | 137MCG/SPRAY | SOLUTION |
PACKAGE FILES


Generic Name
AZELASTINE
Substance Name
AZELASTINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
NASAL
Application Number
ANDA204660
Description
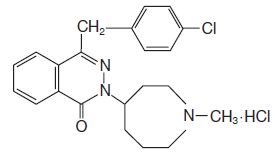
11 DESCRIPTION Azelastine HCl nasal spray, 0.1%, 137 micrograms (mcg), is an antihistamine formulated as a metered-spray solution for intranasal administration. Azelastine HCl, USP occurs as a white, almost odorless, crystalline powder with a bitter taste. It has a molecular weight of 418.37. It is sparingly soluble in water, methanol, and propylene glycol and slightly soluble in ethanol, octanol, and glycerine. It has a melting point of about 225°C and the pH of a saturated solution is between 5.0 and 5.4. Its chemical name is (±)-1-(2H)-phthalazinone,4-[(4-chlorophenyl) methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)-, monohydrochloride. Its molecular formula is C 22 H 24 ClN 3 O•HCl with the following chemical structure: Azelastine HCl nasal spray, 0.1% contains 0.1% azelastine HCl, USP in an aqueous solution at pH 6.8 ± 0.3. It also contains benzalkonium chloride (125 mcg/mL), citric acid, dibasic sodium phosphate, edetate disodium, hypromellose, purified water (pH 6.8) and sodium chloride. After priming [see Dosage and Administration (2.3) ] , each metered spray delivers a 0.137 mL mean volume containing 137 mcg of azelastine HCl, USP (equivalent to 125 mcg of azelastine base). The bottle can deliver 200 metered sprays. 1
How Supplied
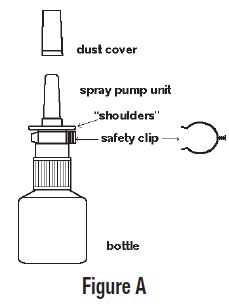
16 HOW SUPPLIED/STORAGE AND HANDLING Azelastine HCl nasal spray 0.1%, 137 mcg, is supplied as a 30-mL package (NDC 65162-676-84) delivering 200 metered sprays in a high-density polyethylene (HDPE) bottle fitted with a metered-dose spray pump unit. The spray pump unit consists of a nasal spray pump fitted with a white safety clip and a white or clear plastic dust cover. The net content of the bottle is 30 mL (net weight 30 g of solution). Each bottle contains 30 mg (1 mg/mL) of azelastine HCl, USP. After priming [see Dosage and Administration (2.3) ] , each spray delivers a fine mist containing a mean volume of 0.137 mL solution containing 137 mcg of azelastine HCl, USP. The correct amount of medication in each spray cannot be assured before the initial priming and after 200 sprays have been used, even though the bottle is not completely empty. The bottle should be discarded after 200 sprays have been used. Azelastine HCl nasal spray, 0.1% should not be used after the expiration date “EXP” printed on the medicine label and carton. Storage: Store upright at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from freezing.
Indications & Usage
1 INDICATIONS AND USAGE Azelastine hydrochloride (HCl) nasal spray, 0.1% is indicated for the treatment of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 5 years and older, and for the treatment of the symptoms of vasomotor rhinitis in adults and adolescent patients 12 years and older. Azelastine HCl nasal spray, 0.1% is an H 1 -receptor antagonist indicated for the treatment of the symptoms of seasonal allergic rhinitis in adults and pediatric patients 5 years and older and for the treatment of the symptoms of vasomotor rhinitis in adults and adolescent patients 12 years and older. (1)
Dosage and Administration
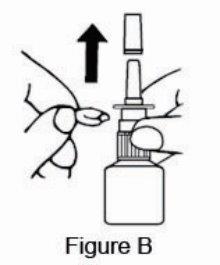
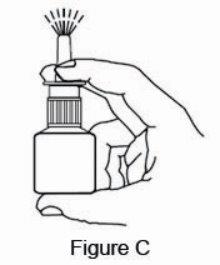
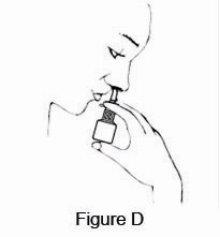
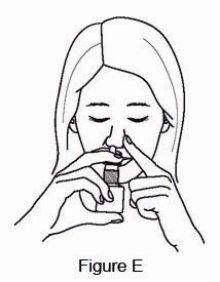
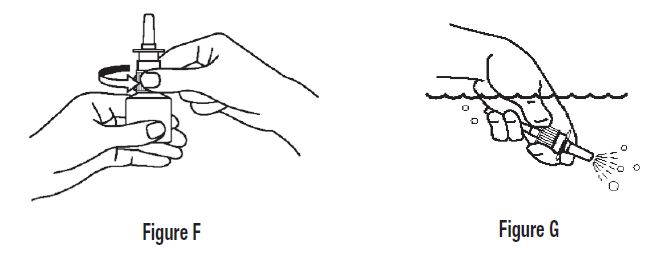
2 DOSAGE AND ADMINISTRATION For intranasal use only. (2.3) Seasonal allergic rhinitis: Pediatric patients 5 to 11 years of age: 1 spray per nostril twice daily. (2.1) Adults and adolescents 12 years of age and older: 1 or 2 sprays per nostril twice daily. (2.1) Vasomotor rhinitis: 2 sprays per nostril twice daily in adults and adolescents 12 years of age and older. (2.2) Prime azelastine HCl nasal spray, 0.1% before initial use and when it has not been used for 3 or more days. (2.3) 2.1 Seasonal Allergic Rhinitis The recommended dosage of azelastine HCl nasal spray, 0.1% in adults and adolescent patients 12 years and older with seasonal allergic rhinitis is one or two sprays per nostril twice daily. The recommended dosage of azelastine HCl nasal spray, 0.1% in pediatric patients 5 years to 11 years of age is one spray per nostril twice daily. 2.2 Vasomotor Rhinitis The recommended dosage of azelastine HCl nasal spray, 0.1% in adults and adolescent patients 12 years and older with vasomotor rhinitis is two sprays per nostril twice daily. 2.3 Important Administration Instructions Administer azelastine HCl nasal spray, 0.1% by the intranasal route only. Priming: Prime azelastine HCl nasal spray, 0.1% before initial use by releasing 4 sprays or until a fine mist appears. When azelastine HCl nasal spray, 0.1% has not been used for 3 or more days, reprime with 2 sprays or until a fine mist appears. Avoid spraying azelastine HCl nasal spray, 0.1% into the eyes.
