Drug Catalog - Product Detail
BROMOCRIPTINE MESYLATE CP 5MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-7096-01 | MYLAN | 100 | 5MG | CAPSULE |
PACKAGE FILES

Generic Name
BROMOCRIPTINE MESYLATE
Substance Name
BROMOCRIPTINE MESYLATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA077226
Description
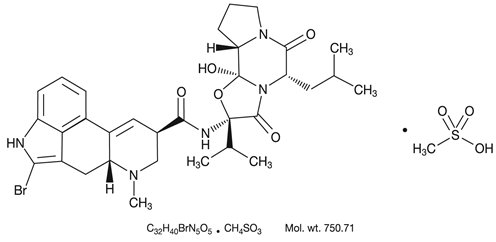
DESCRIPTION Bromocriptine mesylate, USP is an ergot derivative with potent dopamine receptor agonist activity. Each bromocriptine mesylate capsule for oral administration contains 5 mg bromocriptine (as the mesylate). Bromocriptine mesylate is chemically designated as 2-Bromoergocryptine monomethanesulfonate (salt). The structural formula is: The active ingredient in bromocriptine mesylate capsules, USP is bromocriptine mesylate, USP. The inactive ingredients are as follows: anhydrous lactose, black iron oxide, D&C Yellow No. 10 Aluminum Lake, gelatin, magnesium stearate, maleic acid, pregelatinized starch (corn), red iron oxide, sodium lauryl sulfate, titanium dioxide and yellow iron oxide. In addition, the black imprinting ink contains black iron oxide, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, n-butyl alcohol, propylene glycol, SDA 3A alcohol, SD-45 alcohol and shellac glaze. Bromocriptine Mesylate Structural Formula
How Supplied
HOW SUPPLIED Bromocriptine Mesylate Capsules, USP are available containing bromocriptine mesylate, USP equivalent to 5 mg bromocriptine. The 5 mg capsules are hard-shell gelatin capsules with a light brown opaque cap and ivory opaque body filled with a white to off-white powder. The capsules are axially printed with MYLAN over 7096 in black ink on both the cap and body. They are available as follows: NDC 0378-7096-93 bottles of 30 capsules NDC 0378-7096-01 bottles of 100 capsules Store and Dispense: Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A. Manufactured by: Granules India Limited Survey No. 160/A, 161/E, 162, 174/A, Gagillapur Village, Dundigal-Gandimaisamma Mandal, Medchal-Malkajgiri District – 500043, Telangana, INDIA AP/DRUGS/37/2003 Revised: 10/2021 GR:BROMC:R3
Indications & Usage
INDICATIONS AND USAGE Hyperprolactinemia-Associated Dysfunctions Bromocriptine mesylate tablets and capsules are indicated for the treatment of dysfunctions associated with hyperprolactinemia including amenorrhea with or without galactorrhea, infertility or hypogonadism . Bromocriptine treatment is indicated in patients with prolactin-secreting adenomas , which may be the basic underlying endocrinopathy contributing to the above clinical presentations. Reduction in tumor size has been demonstrated in both male and female patients with macroadenomas. In cases where adenectomy is elected, a course of bromocriptine therapy may be used to reduce the tumor mass prior to surgery. Acromegaly Bromocriptine mesylate tablet and capsule therapy is indicated in the treatment of acromegaly. Bromocriptine therapy, alone or as adjunctive therapy with pituitary irradiation or surgery, reduces serum growth hormone by 50% or more in approximately ½ of patients treated, although not usually to normal levels. Since the effects of external pituitary radiation may not become maximal for several years, adjunctive therapy with bromocriptine offers potential benefit before the effects of irradiation are manifested. Parkinson’s Disease Bromocriptine mesylate tablets or capsules are indicated in the treatment of the signs and symptoms of idiopathic or postencephalitic Parkinson’s disease. As adjunctive treatment to levodopa (alone or with a peripheral decarboxylase inhibitor), bromocriptine therapy may provide additional therapeutic benefits in those patients who are currently maintained on optimal dosages of levodopa, those who are beginning to deteriorate (develop tolerance) to levodopa therapy, and those who are experiencing “end of dose failure” on levodopa therapy. Bromocriptine therapy may permit a reduction of the maintenance dose of levodopa and, thus may ameliorate the occurrence and/or severity of adverse reactions associated with long-term levodopa therapy such as abnormal involuntary movements (e.g., dyskinesias) and the marked swings in motor function (“on-off” phenomenon). Continued efficacy of bromocriptine therapy during treatment of more than 2 years has not been established. Data are insufficient to evaluate potential benefit from treating newly diagnosed Parkinson’s disease with bromocriptine. Studies have shown, however, significantly more adverse reactions (notably nausea, hallucinations, confusion and hypotension) in bromocriptine-treated patients than in levodopa/carbidopa-treated patients. Patients unresponsive to levodopa are poor candidates for bromocriptine therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION General It is recommended that bromocriptine mesylate tablets and capsules be taken with food. Patients should be evaluated frequently during dose escalation to determine the lowest dosage that produces a therapeutic response. Hyperprolactinemic Indications The initial dosage of bromocriptine mesylate tablets in adults is one ½ to one 2.5 mg scored tablet daily. An additional 2.5 mg tablet may be added to the treatment regimen as tolerated every 2 to 7 days until an optimal therapeutic response is achieved. The therapeutic dosage ranged from 2.5-15 mg daily in adults studied clinically. Based on limited data in children of age 11 to 15, (see Pediatric Use ) the initial dose is one ½ to one 2.5 mg scored tablet daily. Dosing may need to be increased as tolerated until a therapeutic response is achieved. The therapeutic dosage ranged from 2.5-10 mg daily in children with prolactin-secreting pituitary adenomas. In order to reduce the likelihood of prolonged exposure to bromocriptine mesylate tablets or capsules should an unsuspected pregnancy occur, a mechanical contraceptive should be used in conjunction with bromocriptine therapy until normal ovulatory menstrual cycles have been restored. Contraception may then be discontinued in patients desiring pregnancy. Thereafter, if menstruation does not occur within 3 days of the expected date, bromocriptine therapy should be discontinued and a pregnancy test performed. Acromegaly Virtually all acromegalic patients receiving therapeutic benefit from bromocriptine mesylate tablets and capsules also have reductions in circulating levels of growth hormone. Therefore, periodic assessment of circulating levels of growth hormone will, in most cases, serve as a guide in determining the therapeutic potential of bromocriptine. If, after a brief trial with bromocriptine therapy, no significant reduction in growth hormone levels has taken place, careful assessment of the clinical features of the disease should be made, and if no change has occurred, dosage adjustment or discontinuation of therapy should be considered. The initial recommended dosage is one ½ to one 2.5 mg bromocriptine mesylate tablet on retiring (with food) for 3 days. An additional one ½ to 1 bromocriptine mesylate tablet should be added to the treatment regimen as tolerated every 3 to 7 days until the patient obtains optimal therapeutic benefit. Patients should be reevaluated monthly and the dosage adjusted based on reductions of growth hormone or clinical response. The usual optimal therapeutic dosage range of bromocriptine mesylate tablets and capsules varies from 20-30 mg/day in most patients. The maximal dosage should not exceed 100 mg/day. Patients treated with pituitary irradiation should be withdrawn from bromocriptine therapy on a yearly basis to assess both the clinical effects of radiation on the disease process as well as the effects of bromocriptine therapy. Usually a 4 to 8 week withdrawal period is adequate for this purpose. Recurrence of the signs/symptoms or increases in growth hormone indicate the disease process is still active and further courses of bromocriptine should be considered. Parkinson’s Disease The basic principle of bromocriptine mesylate therapy is to initiate treatment at a low dosage and, on an individual basis, increase the daily dosage slowly until a maximum therapeutic response is achieved. The dosage of levodopa during this introductory period should be maintained, if possible. The initial dose of bromocriptine is one ½ of a 2.5 mg tablet twice daily with meals. Assessments are advised at 2-week intervals during dosage titration to ensure that the lowest dosage producing an optimal therapeutic response is not exceeded. If necessary, the dosage may be increased every 14 to 28 days by 2.5 mg/day with meals. Should it be advisable to reduce the dosage of levodopa because of adverse reactions, the daily dosage of bromocriptine, if increased, should be accomplished gradually in small (2.5 mg) increments. The safety of bromocriptine mesylate tablets and capsules has not been demonstrated in dosages exceeding 100 mg/day.
