Drug Catalog - Product Detail
CARBINOXAMINE MALEATE TB 4MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 51991-0333-01 | BRECKENRIDGE | 100 | 4MG | TABLET |
PACKAGE FILES

Generic Name
CARBINOXAMINE MALEATE
Substance Name
CARBINOXAMINE MALEATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA040442
Description
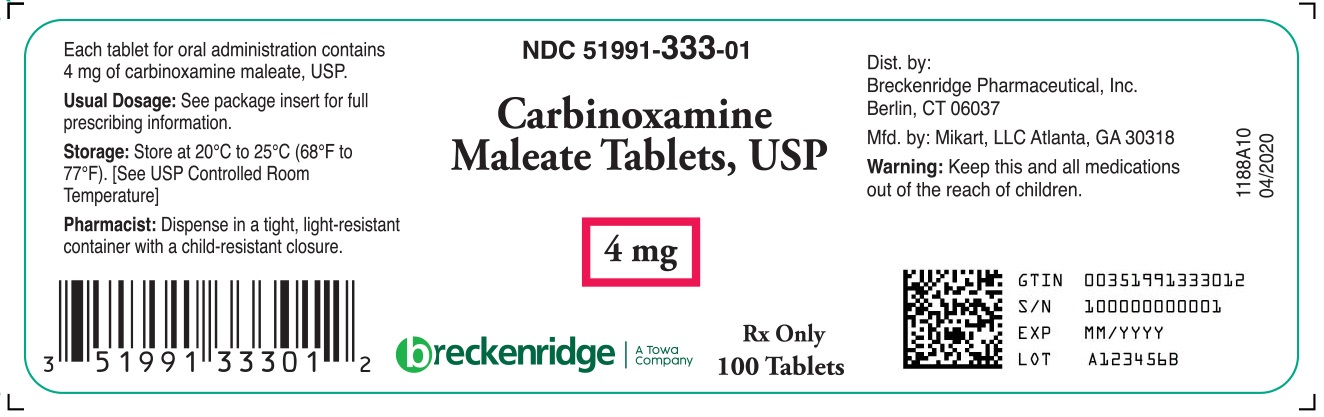
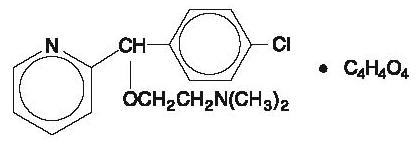
DESCRIPTION Carbinoxamine maleate is a histamine-H 1 receptor blocking agent. Each tablet contains 4 mg carbinoxamine maleate and the following inactive ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. Each 5 mL (teaspoonful) of oral solution contains 4 mg carbinoxamine maleate and the following inactive ingredients: artificial bubble gum flavor, citric acid (anhydrous), glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate (hydrous) and sorbitol solution. Carbinoxamine maleate is freely soluble in water. Its structure is: 2-[(4-chlorophenyl)-2-pyridinylmethoxy]- N , N -dimethylethanamine (Z)-2-butenedioate (1:1) C 16 H 19 CIN 2 O∙C 4 H 4 O 4 MW = 406.86 carbinoxamine maleate chemical structure
How Supplied
HOW SUPPLIED Carbinoxamine Maleate Tablets, USP 4 mg are supplied as white, round, scored tablets, debossed “109” on one side and score over “C” on the other side, and is supplied in bottles of 100 tablets, NDC 51991-333-01. Carbinoxamine Maleate Oral Solution, 4 mg/5 mL is supplied as clear, colorless liquid with a bubble gum aroma, and is supplied in 4 fl. oz. bottles, NDC 51991-334-04.
Indications & Usage
INDICATIONS AND USAGE Carbinoxamine maleate is effective for the symptomatic treatment of: Seasonal and perennial allergic rhinitis. Vasomotor rhinitis. Allergic conjunctivitis due to inhalant allergens and foods. Mild, uncomplicated allergic skin manifestations of urticaria and angioedema. Dermatographism. As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled. Amelioration of the severity of allergic reactions to blood or plasma.
Dosage and Administration
DOSAGE AND ADMINISTRATION Carbinoxamine maleate is contraindicated in children younger than 2 years of age (see CONTRAINDICATIONS ). Carbinoxamine maleate should be taken on an empty stomach with water. DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT. Carbinoxamine maleate dosage should be based on the severity of the condition and the response of the patient. The drug is well tolerated in adults in doses as high as 24 mg daily, in divided doses, over prolonged periods. On the other hand, some patients respond to as little as 4 mg daily. Clinical experience suggests the following dosage schedules: Tablets Usual Adult Dosage: 1 or 2 tablets (4 to 8 mg) 3 to 4 times daily Usual Child’s Dosage: Six to eleven years – 1/2 to 1 tablet (2 to 4 mg) 3 to 4 times daily. Oral Solution Usual Adult Dosage: 1 or 2 teaspoonfuls (4 to 8 mg) 3 to 4 times daily Usual Child's Dosage (approximately 0.2 to 0.4 mg/kg/day, divided into 3 to 4 doses): Six to eleven years - ½ to 1 teaspoonful (2 to 4 mg) 3 to 4 times daily. Dosing for children 2 to 5 years of age should be based on weight whenever possible. The usual dosage for children 2 to 5 years of age is approximately 0.2 to 0.4 mg/kg/day, divided into 3 to 4 daily doses. In general, this corresponds to a dose of 1/4 to 1/2 teaspoonful (1 to 2 mg) 3 to 4 times daily.
