Drug Catalog - Product Detail
CEFACLOR CAP 250 MG 30 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61442-0171-30 | CARLSBAD TECHNOLOGIES | 30 | 250MG | CAPSULE |
PACKAGE FILES

Generic Name
CEFACLOR
Substance Name
CEFACLOR
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA065146
Description
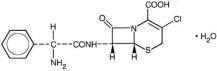
DESCRIPTION Cefaclor, USP is a semisynthetic cephalosporin antibiotic for oral administration. It is chemically designated as 3-chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate. The chemical formula for cefaclor is C 15 H 14 ClN 3 O 4 S•H 2 O and the molecular weight is 385.82. Each 250-mg capsule contains cefaclor monohydrate equivalent to 250 mg (0.68 mmol) of anhydrous cefaclor and inactive ingredients: magnesium stearate, sodium starch glycolate, lactose monohydrate, talc. The 250 mg capsule shell contains gelatin, titanium dioxide, FD & C Blue No. 1, FD & C Red No. 3, and imprinting ink components: shellac, strong ammonia solution, potassium hydroxide, black iron oxide, .dehydrated alcohol, isopropyl alcohol, butyl alcohol and propylene glycol. Each 500-mg capsule contains cefaclor monohydrate equivalent to 500 mg (1.36 mmol) of anhydrous cefaclor and inactive ingredients: magnesium stearate, sodium starch glycolate, lactose monohydrate, talc. The 500 mg capsule shell contains gelatin, titanium dioxide, FD & C Blue No. 1, FD & C Red No. 3, FD & C Yellow No. 6, FD & C Red No. 40, and imprinting ink components: shellac, strong ammonia solution, titanium dioxide, FD & C Blue No. 1 aluminum lake, dehydrated alcohol, isopropyl alcohol, butyl alcohol and propylene glycol. image description
How Supplied
HOW SUPPLIED Cefaclor Capsule USP 250 mg (blue cap and pink body hard gelatin capsule containing white to slightly yellowish powder imprinted with ”KRC” on both capsule cap and capsule body) contains cefaclor USP (monohydrate) equivalent to 250 mg anhydrous cefaclor. Bottle of 30 NDC 61442-171-30 Bottle of 100 NDC 61442-171-01 Bottle of 500 NDC 61442-171-05 Cefaclor Capsule USP 500 mg (blue cap and orange body hard gelatin capsule containing white to slightly yellowish powder imprinted with “KRC500” on both capsule cap and capsule body) contains cefaclor USP (monohydrate) equivalent to 500 mg anhydrous cefaclor. Bottle of 30 NDC 61442-172-30 Bottle of 100 NDC 61442-172-01 Bottle of 500 NDC 61442-172-05 Store at 20℃ - 25℃ (68℃ to 77℉) [See USP Controlled Room Temperature].
Indications & Usage
INDICATIONS AND USAGE Cefaclor is indicated in the treatment of the following infections when caused by susceptible strains of the designated microorganisms: Otitis media caused by Streptococcus pneumoniae, Haemophilus influenzae, staphylococci, and Streptococcus pyogenes Note: β-lactamase-negative, ampicillin-resistant (BLNAR) strains of Haemophilus influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility of some BLNAR strains. Lower respiratory tract infections, including pneumonia, caused by Streptococcus pneumoniae, Haemophilus influenzae, and Streptococcus pyogenes Note: β-lactamase-negative, ampicillin-resistant (BLNAR) strains of Haemophilus influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility of some BLNAR strains. Pharyngitis and Tonsillitis, caused by Streptococcus pyogenes Note: Penicillin is the usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. Cefaclor is generally effective in the eradication of streptococci from the nasopharynx; however, substantial data establishing the efficacy of cefaclor in the subsequent prevention of rheumatic fever are not available at present. Urinary tract infections, including pyelonephritis and cystitis, caused by Escherichia coli, Proteus mirabilis, Klebsiella spp ., and coagulase-negative staphylococci Skin and skin structure infections caused by Staphylococcus aureus and Streptococcus pyogenes Appropriate culture and susceptibility studies should be performed to determine susceptibility of the causative organism to cefaclor. To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefaclor Capsule and other antibacterial drugs, Cefaclor Capsule should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
DOSAGE AND ADMINISTRATION Cefaclor is administered orally. Adults ─ The usual adult dosage is 250 mg every 8 hours. For more severe infections (such as pneumonia) or those caused by less susceptible organisms, doses may be doubled. Pediatric Patients ─ The usual recommended daily dosage for pediatric patients is 20 mg/kg/day in divided doses every 8 hours. In more serious infections, otitis media, and infections caused by less susceptible organisms, 40 mg/kg/day are recommended, with a maximum dosage of 1 g/day. Cefaclor may be administered in the presence of impaired renal function. Under such a condition, the dosage usually is unchanged (see PRECAUTIONS ). In the treatment of β-hemolytic streptococcal infections, a therapeutic dosage of cefaclor should be administered for at least 10 days.
