Drug Catalog - Product Detail
CINACALCET HCL 30MG TB 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 67877-0503-30 | ASCEND LABORATORIES | 30 | 30MG | TABLET |
PACKAGE FILES





Generic Name
CINACALCET
Substance Name
CINACALCET HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA210570
Description
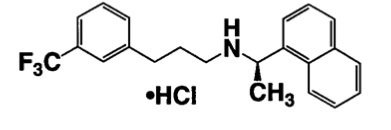
11 DESCRIPTION Cinacalcet tablets contain the hydrochloride salt of the active ingredient cinacalcet, a positive modulator of the calcium sensing receptor. The empirical formula for cinacalcet is C 22 H 22 F 3 N⋅HCl with a molecular weight of 393.9 g/mol (hydrochloride salt) and 357.4 g/mol (free base). It has one chiral center having an R-absolute configuration. The R-enantiomer is the more potent enantiomer and has been shown to be responsible for pharmacodynamic activity. The hydrochloride salt of cinacalcet is a white to off-white, powder that is soluble in methanol, 95% ethanol and slightly soluble in water. Cinacalcet tablets are formulated as light-green, film-coated, oval-shaped tablets for oral administration in strengths of 30 mg, 60 mg, and 90 mg of cinacalcet as the free base equivalent (33 mg, 66 mg, and 99 mg as the hydrochloride salt, respectively). The hydrochloride salt of cinacalcet is described chemically as N-[1-(R)-(-)-(1-naphthyl)ethyl]-3-[3-(trifluoromethyl)phenyl]-1-aminopropane hydrochloride and has the following structural formula: Inactive Ingredients The following are the inactive ingredients in cinacalcet tablets: Lactose monohydrate, microcrystalline cellulose (GR 101 and GR 102), crospovidone, hydroxypropyl cellulose, magnesium stearate. In addition polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, iron oxide yellow, FD&C blue #2/Indigo carmine AL are included in the film coating. cinacalcet-str
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Cinacalcet Tablets 30 mg are formulated as light green colored, oval shaped, film coated tablets debossed with “A33” on one side and “30” on other side, packaged in- bottles of 30 tablets (NDC 67877-503-30), bottles of 100 tablets (NDC 67877-503-01) and carton of 10 tablets (1x10 Unit-dose) (NDC 67877-503-33) Cinacalcet Tablets 60 mg are formulated as light green colored, oval shaped, film coated tablets debossed with “A34” on one side and “60” on other side, packaged in- bottles of 30 tablets (NDC 67877-504-30), bottles of 100 tablets (NDC 67877-504-01) and carton of 10 tablets (1x10 Unit-dose) (NDC 67877-504-33) Cinacalcet Tablets 90 mg are formulated as light green colored, oval shaped, film coated tablets debossed with “A35” on one side and “90” on other side, packaged in- bottles of 30 tablets (NDC 67877-505-30), bottles of 100 tablets (NDC 67877-505-01) and carton of 10 tablets (1x10 Unit-dose) (NDC 67877-505-33) Storage Store at 25ºC (77ºF); excursions permitted from 15°C to 30ºC (59°F to 86ºF). [See USP Controlled Room Temperature].
Indications & Usage
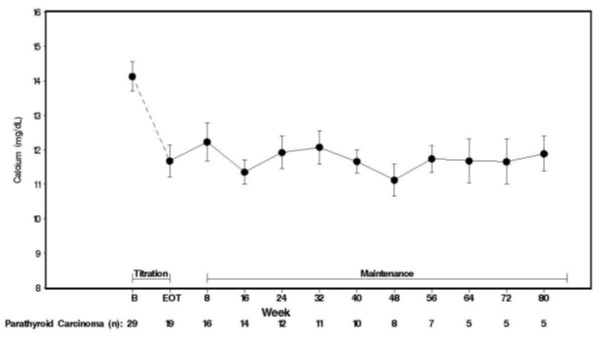
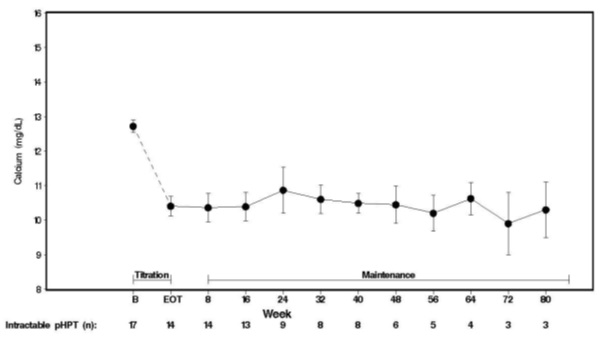
1 INDICATIONS & USAGE Cinacalcet is a positive modulator of the calcium sensing receptor indicated for: Secondary Hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis. (1.1) Limitations of Use: Cinacalcet is not indicated for use in patients with CKD who are not on dialysis. Hypercalcemia in adult patients with Parathyroid Carcinoma (PC). (1.2) Severe hypercalcemia in adult patients with primary HPT who are unable to undergo parathyroidectomy. (1.3) 1.1 Secondary Hyperparathyroidism Cinacalcet are indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on dialysis [see Clinical Studies ( 14.1 )]. Limitations of Use: Cinacalcet is not indicated for use in adult patients with CKD who are not on dialysis because of an increased risk of hypocalcemia [see Warnings and Precautions ( 5.1 )]. 1.2 Parathyroid Carcinoma Cinacalcet is indicated for the treatment of hypercalcemia in adult patients with Parathyroid Carcinoma [see Clinical Studies ( 14.2) ] . 1.3 Primary Hyperparathyroidism Cinacalcet are indicated for the treatment of severe hypercalcemia in adult patients with primary HPT who are unable to undergo parathyroidectomy [ see Clinical Studies (14.3) ]
Dosage and Administration
2 DOSAGE & ADMINISTRATION Cinacalcet tablets should be taken with food or shortly after a meal (2.1). Tablets should always be taken whole and not divided (2.1) Secondary HPT in patients with CKD on dialysis (2.2) : Starting dose is 30 mg once daily. Titrate dose no more frequently than every 2 to 4 weeks through sequential doses of 30, 60, 90, 120, and 180 mg once daily as necessary to achieve targeted intact parathyroid hormone (iPTH) levels. iPTH levels should be measured no earlier than 12 hours after most recent dose. Hypercalcemia in Patients with PC or severe hypercalcemia in patients with primary HPT (2.3). Starting dose is 30 mg twice daily. Titrate dose every 2 to 4 weeks through sequential doses of 30 mg twice daily, 60 mg twice daily, 90 mg twice daily,and 90 mg three or four times daily as necessary to normalize serum calcium levels. Once the maintenance dose has been established, monitor serum calcium approximately monthly for patients with secondary HPT and every 2 months for patients with PC or primary HPT (2.4) 2.1 Administration Cinacalcet Tablets should be taken with food or shortly after a meal. Cinacalcet Tablets are administered orally and should always be taken whole and not chewed, crushed, or divided. 2.2 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis The recommended starting oral dose of cinacalcet is 30 mg once daily. Serum calcium and serum phosphorus should be measured within 1 week and intact parathyroid hormone (iPTH) should be measured 1 to 4 weeks after initiation or dose adjustment of cinacalcet [see Dosage and Administration (2.3)] . Cinacalcet should be titrated no more frequently than every 2 to 4 weeks through sequential doses of 30, 60, 90, 120, and 180 mg once daily to target iPTH levels of 150 to 300 pg/mL. Serum iPTH levels should be assessed no earlier than 12 hours after dosing with cinacalcet . Cinacalcet can be used alone or in combination with vitamin D sterols and/or phosphate binders. During dose titration, serum calcium levels should be monitored frequently and if levels decrease below the normal range, appropriate steps should be taken to increase serum calcium levels, such as by providing supplemental calcium, initiating or increasing the dose of calcium-based phosphate binder,initiating or increasing the dose of vitamin D sterols, or temporarily withholding treatment with cinacalcet [see Dosage and Administration ( 2.4 ) and Warnings and Precautions (5.1)] . 2.3 Patients with Parathyroid Carcinoma and Primary Hyperparathyroidism The recommended starting oral dose of cinacalcet is 30 mg twice daily. The dose of cinacalcet should be titrated every 2 to 4 weeks through sequential doses of 30 mg twice daily, 60 mg twice daily, and 90 mg twice daily, and 90 mg 3 or 4 times daily as necessary to normalize serum calcium levels. Serum calcium should be measured within 1 week after initiation or dose adjustment of cinacalcet [ see Dosage and Administration (2.4) and Warnings and Precautions (5.1)]. 2.4 Switching from Parsabiv (etelcalcetide) to cinacalcet Discontinue etelcalcetide for at least 4 weeks prior to starting cinacalcet. Ensure corrected serum calcium is at or above the lower limit of normal prior to cinacalcet initiation. [see Warnings and Precautions (5.1)] . Initiate cinacalcet treatment at a starting dose of 30 mg once daily. 2.5 Monitoring for Hypocalcemia Once the maintenance dose has been established, serum calcium should be measured approximately monthly for patients with secondary hyperparathyroidism with CKD on dialysis, and every 2 months for patients with parathyroid carcinoma or primary hyperparathyroidism [ see Dosage and Administration (2.2 , 2.3)] . For secondary hyperparathyroidism patients with CKD on dialysis, if serum calcium falls below 8.4 mg/dL but remains above 7.5 mg/dL, or if symptoms of hypocalcemia occur, calcium-containing phosphate binders and/or vitamin D sterols can be used to raise serum calcium. If serum calcium falls below 7.5 mg/dL, or if symptoms of hypocalcemia persist and the dose of vitamin D cannot be increased, withhold administration of cinacalcet until serum calcium levels reach 8.0 mg/dL and/or symptoms of hypocalcemia have resolved. Treatment should be reinitiated using the next lowest dose of cinacalcet [see Dosage and Administration (2.2)] .
