Drug Catalog - Product Detail
CLOBETASOL PROPIONATE 0.05% CREAM 15GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 70700-0109-15 | XIROMED | 15 | 0.05% | CREAM |
PACKAGE FILES

Generic Name
CLOBETASOL PROPIONATE
Substance Name
CLOBETASOL PROPIONATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA210034
Description
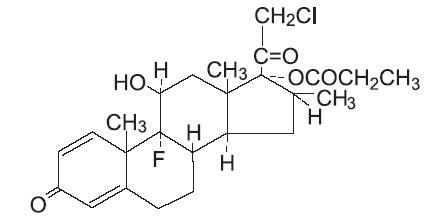
DESCRIPTION Clobetasol Propionate Cream USP, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid activity. Chemically, clobetasol propionate is (11ß,16ß)-21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-pregna-1,4- diene-3,20-dione, and it has the following structural formula: Clobetasol propionate has the molecular formula C 25 H 32 CIFO 5 and a molecular weight of 467. It is a white to cream-colored crystalline powder insoluble in water. Clobetasol propionate cream contains clobetasol propionate 0.5 mg/g in a cream base composed of cetyl alcohol, citric acid monohydrate, glycol stearate, lanolin liquid, methylparaben, PEG-400 stearate, polysorbate 60, propylene glycol, propylparaben, purified water, stearyl alcohol, and white petrolatum. Sodium hydroxide may be used to adjust pH. Clobetasol Propionate Structural Formula
How Supplied
HOW SUPPLIED Clobetasol Propionate Cream, USP, 0.05% is supplied in 15-g tubes (NDC 70700-109-15), 30-g tubes (NDC 70700-109-16), 45-g tubes (NDC 70700-109-18), and 60-g tubes (NDC 70700-109-17). Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Clobetasol propionate cream should not be refrigerated. Rx only Distributed by: Xiromed, LLC Florham Park, NJ 07932 Made in Spain Rev. 03/2018 PI-109-00
Indications & Usage
INDICATIONS AND USAGE Clobetasol propionate cream is a super-high potency corticosteroid formulation indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Treatment beyond 2 consecutive weeks is not recommended, and the total dosage should not exceed 50 g per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Use in pediatric patients under 12 years of age is not recommended. As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
Dosage and Administration
DOSAGE AND ADMINISTRATION Apply a thin layer of clobetasol propionate cream to the affected skin areas twice daily and rub in gently and completely (see INDICATIONS AND USAGE ). Clobetasol propionate cream is a super-high potency topical corticosteroids; therefore, treatment should be limited to 2 consecutive weeks and amounts greater than 50 g/week should not be used. As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. Clobetasol propionate cream should not be used with occlusive dressings. Geriatric Use In studies where geriatric patients (65 years of age or older, see PRECAUTIONS ) have been treated with clobetasol propionate cream, safety did not differ from that in younger patients; therefore, no dosage adjustment is recommended.
