Drug Catalog - Product Detail
Clobetasol Propionate 0.05% Spray 125 ml
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0480-56 | GLENMARK PHARMACEUTICALS | 125 | 0.05% | LIQUID |
PACKAGE FILES





Generic Name
CLOBETASOL PROPIONATE
Substance Name
CLOBETASOL PROPIONATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA209004
Description
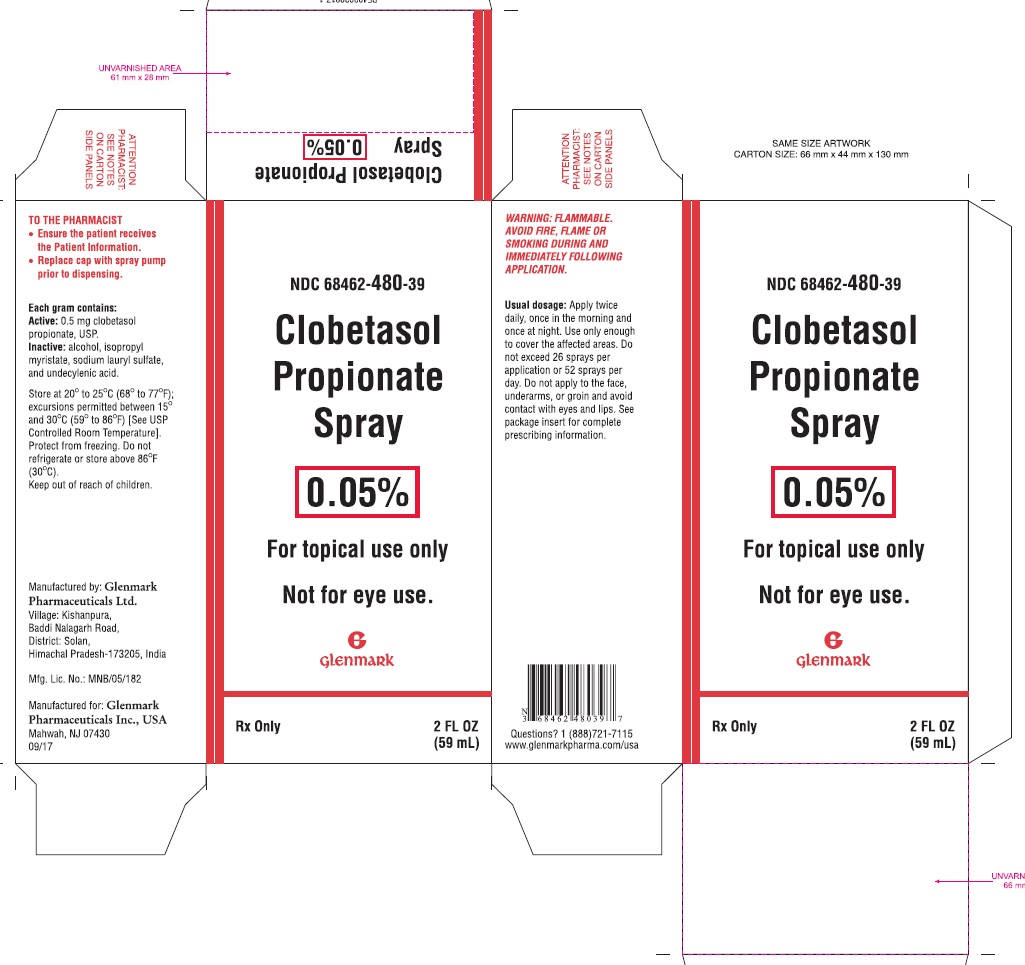
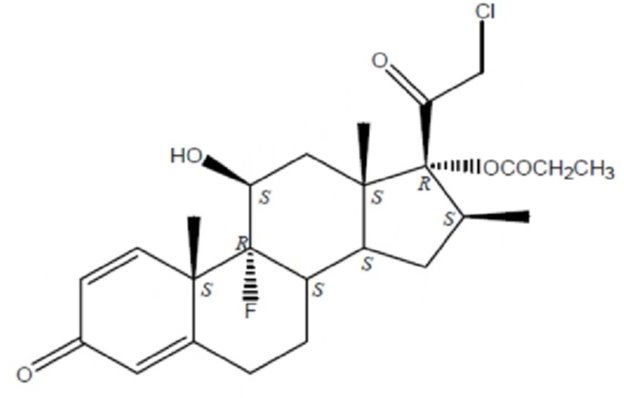
11 DESCRIPTION Clobetasol Propionate Spray, 0.05% contains clobetasol propionate USP, a synthetic fluorinated corticosteroid, for topical use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents. Clobetasol propionate, USP is 21-chloro-9-fluoro-11β,17-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17-propionate, with the empirical formula C 25 H 32 ClFO 5, and a molecular weight of 466.97 g/mol (CAS Registry Number 25122-46-7). The following is the chemical structure: Clobetasol propionate, USP Clobetasol propionate, USP is a white to almost white, crystalline powder, practically insoluble in water, slightly soluble in benzene and diethyl ether; sparingly soluble in ethanol; freely soluble in acetone, in dimethylsulfoxide, in chloroform, in methanol and in dioxane. Each gram of Clobetasol Propionate Spray, 0.05% contains 0.5 mg of clobetasol propionate, in a clear, colorless liquid composed of alcohol, isopropyl myristate, sodium lauryl sulfate, and undecylenic acid. Structure.jpg
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Clobetasol Propionate Spray, 0.05% is a clear, colorless liquid, supplied in a white HDPE bottle with a white polypropylene cap and white LDPE liner in the following sizes: 2 fl oz/59 mL NDC 68462-480-39 4.25 fl oz/125 mL NDC 68462-480-56 Storage: Keep tightly closed. Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Do not freeze, refrigerate or store above 86°F (30°C). Spray is flammable; avoid heat, flame or smoking when using this product.
Indications & Usage
1 INDICATIONS AND USAGE Clobetasol propionate spray, 0.05% is a corticosteroid indicated for the topical treatment of moderate to severe plaque psoriasis affecting up to 20% body surface area (BSA) in patients 18 years of age or older. ( 1.1 ) Limitations of Use: • Do not use on the face, axillae or groin. ( 1.2 ) • Do not use if atrophy is present at the treatment site. ( 1.2 ) • Do not use for rosacea or perioral dermatitis. ( 1.2 ) 1.1 Indication Clobetasol propionate spray, 0.05% is a super-high potent topical corticosteroid formulation indicated for the treatment of moderate to severe plaque psoriasis affecting up to 20% body surface area (BSA) in patients 18 years of age or older. Patients should be instructed to use clobetasol propionate spray, 0.05% for the minimum amount of time necessary to achieve the desired results [ see Dosage and Administration ( 2 ) ]. Use in patients under 18 years of age is not recommended because safety has not been established and because numerically high rates of HPA axis suppression were seen with other clobetasol propionate topical formulations formulations [see Use in Specific Populations ( 8.4 )]. 1.2 Limitations of Use Clobetasol propionate spray, 0.05% should not be used on the face, axillae, or groin. Clobetasol propionate spray, 0.05% should not be used if there is atrophy at the treatment site. Clobetasol propionate spray, 0.05% should not be used in the treatment of rosacea or perioral dermatitis.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Clobetasol propionate spray, 0.05% is for topical use only, and not for ophthalmic, oral or intravaginal use. Clobetasol propionate spray, 0.05% should be sprayed directly onto the affected skin areas twice daily and rubbed in gently and completely. The total dosage should not exceed 50 g (59 mL or 2 fluid ounces) per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Do not use more than 26 sprays per application or 52 sprays per day. Clobetasol propionate spray, 0.05% contains a topical corticosteroid; therefore treatment should be limited to 4 weeks. Therapy should be discontinued when control has been achieved. Treatment beyond 2 weeks should be limited to localized lesions of moderate to severe plaque psoriasis that have not sufficiently improved after the initial 2 weeks of treatment with clobetasol propionate spray, 0.05%. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. Before prescribing for more than 2 weeks, any additional benefits of extending treatment to 4 weeks should be weighed against the risk of HPA axis suppression. Unless directed by physician, clobetasol propionate spray, 0.05% should not be used with occlusive dressings. • Not for oral, ophthalmic, or intravaginal use. ( 1.2 ) • Spray directly onto the affected skin areas twice daily and rub in gently. ( 2 ) • The total dosage should not exceed 50 g (59 mL or 2 fluid ounces) per week. Do not use more than 26 sprays per application or 52 sprays per day. ( 2 ) • Treatment beyond 2 weeks should be limited to localized lesions of moderate to severe plaque psoriasis that have not sufficiently improved after the initial 2 weeks of treatment with clobetasol propionate spray, 0.05%. ( 2 ) • Do not use for more than 4 weeks ( 2 )
