Drug Catalog - Product Detail
CLONIDINE HCL FOR INJECTION INJECT. 0.5MG/ML 1X10ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00143-9723-01 | HIKMA | 10 | 500MCG/ML | SOLUTION |
PACKAGE FILES



Generic Name
CLONIDINE HYDROCHLORIDE
Substance Name
CLONIDINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAVENOUS
Application Number
ANDA200300
Description
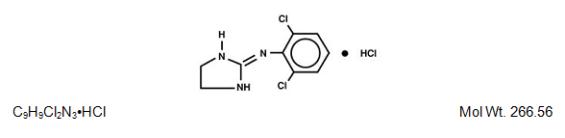
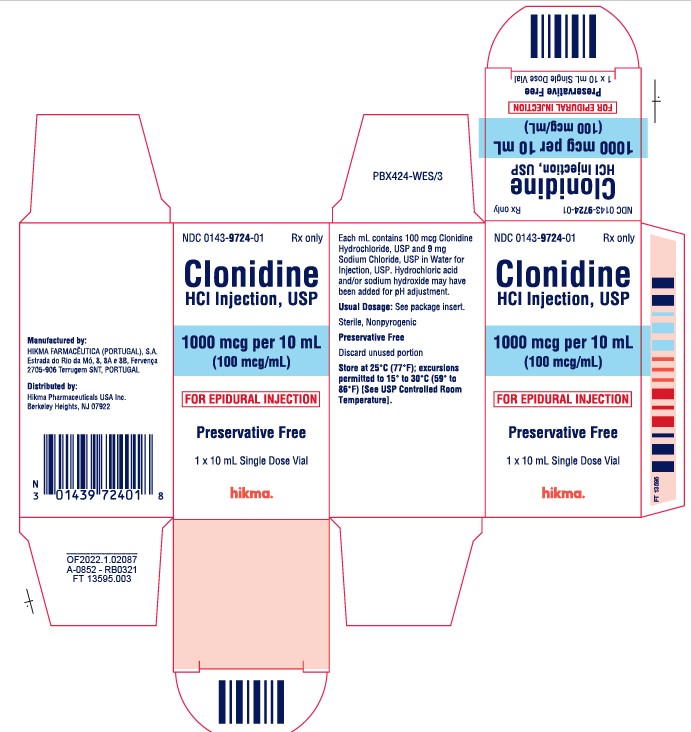
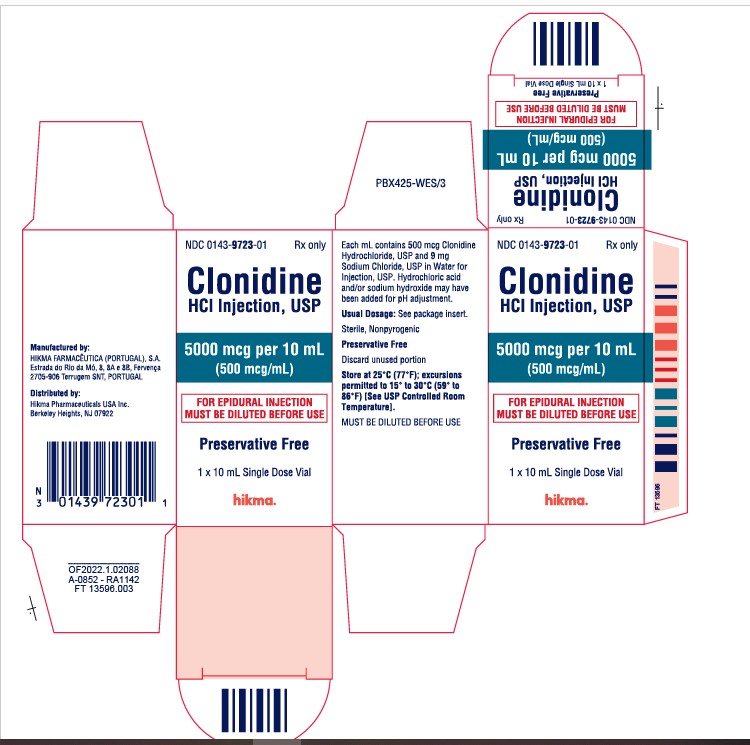
DESCRIPTION Clonidine Hydrochloride Injection, USP is a centrally-acting analgesic solution for use in continuous epidural infusion devices. Clonidine Hydrochloride, USP, is an imidazoline derivative and exists as a mesomeric compound. The chemical names are Benzenamine, 2, 6-dichloro-N-2-imidazolidinylidene monohydrochloride and 2-[(2,6-dichlorophenyl) imino]imidazolidine monohydrochloride. The following is the structural formula: Clonidine Hydrochloride Injection, USP is supplied as a clear, colorless, preservative-free, pyrogen-free, aqueous sterile solution (pH 5 to 7) in 10 mL single-dose vials. Each mL of the 1000 mcg/10 mL (0.1 mg/mL) concentration contains 100 mcg of Clonidine Hydrochloride, USP and 9 mg Sodium Chloride, USP in Water for Injection, USP. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. Each 10 mL vial contains 1 mg (1000 mcg) of clonidine hydrochloride. Each mL of the 5000 mcg/10 mL (0.5 mg/mL) concentration contains 500 mcg of Clonidine Hydrochloride, USP and 9 mg Sodium Chloride, USP in Water for Injection, USP. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. Each 10 mL vial contains 5 mg (5000 mcg) of clonidine hydrochloride. Clonidine hydrochloride structural formula
How Supplied
HOW SUPPLIED NDC 0143-9724-01, 100 mcg/mL solution in 10 mL vials, packaged individually. NDC 0143-9723-01, 500 mcg/mL solution in 10 mL vials, packaged individually. Store at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature]. Preservative Free. Discard unused portion. Manufactured by: HIKMA FARMACÊUTICA (PORTUGAL), S.A. Estrada do Rio da Mó, 8, 8A e 8B – Fervença 2705 – 906 Terrugem SNT PORTUGAL Distributed by: Hikma Pharmaceuticals USA Inc. Berkeley Heights, NJ 07922 Revised: May 2022 PIN265-WES/2
Indications & Usage
INDICATIONS AND USAGE Clonidine Hydrochloride Injection, USP is indicated in combination with opiates for the treatment of severe pain in cancer patients that is not adequately relieved by opioid analgesics alone. Epidural clonidine is more likely to be effective in patients with neuropathic pain than somatic or visceral pain (see CLINICAL PHARMACOLOGY: Clinical Trials ). The safety of this drug product has only been established in a highly selected group of cancer patients, and only after an adequate trial of opioid analgesia. Other use is of unproven safety and is not recommended. In a rare patient, the potential benefits may outweigh the known risks (see WARNINGS ).
Dosage and Administration
DOSAGE AND ADMINISTRATION The recommended starting dose of clonidine hydrochloride injection for continuous epidural infusion is 30 mcg/hr. Although dosage may be titrated up or down depending on pain relief and occurrence of adverse events, experience with dosage rates above 40 mcg/hr is limited. Familiarization with the continuous epidural infusion device is essential. Patients receiving epidural clonidine from a continuous infusion device should be closely monitored for the first few days to assess their response. The 500 mcg/mL (0.5 mg/mL) strength product must be diluted prior to use in 0.9% Sodium Chloride for Injection, USP, to a final concentration of 100 mcg/mL: Volume of Clonidine Hydrochloride Injection 500 mcg/mL Volume of 0.9% Sodium Chloride for Injection, USP Resulting Final Clonidine Hydrochloride Injection Concentration (100 mcg/mL) 1 mL 4 mL 500 mcg/5 mL 2 mL 8 mL 1000 mcg/10 mL 3 mL 12 mL 1500 mcg/15 mL 4 mL 16 mL 2000 mcg/20 mL 5 mL 20 mL 2500 mcg/25 mL 6 mL 24 mL 3000 mcg/ 30 mL 7 mL 28 mL 3500 mcg/ 35 mL 8 mL 32 mL 4000 mcg/40 mL 9 mL 36 mL 4500 mcg/45 mL 10 mL 40 mL 5000 mcg/50 mL Renal Impairment: Dosage should be adjusted according to the degree of renal impairment, and patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis. Clonidine hydrochloride injection must not be used with a preservative. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
