Drug Catalog - Product Detail
CLOPIDOGREL BI SULFATE 75MG TB 500
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 50228-0124-05 | SCIEGEN PHARMACEUTICALS | 500 | 75MG | TABLET |
PACKAGE FILES










Generic Name
CLOPIDOGREL BISULFATE
Substance Name
CLOPIDOGREL BISULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204165
Description
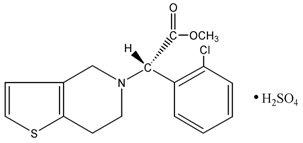
11 DESCRIPTION Clopidogrel bisulfate is a thienopyridine class inhibitor of P2Y 12 ADP platelet receptors. Chemically it is methyl (+)-( S )-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4 H )-acetate sulfate (1:1). The empirical formula of clopidogrel bisulfate is C 16 H 16 ClNO 2 S•H 2 SO 4 and its molecular weight is 419.9. The structural formula is as follows: Clopidogrel bisulfate, USP is a white to off-white powder. It is freely soluble in methanol, practically insoluble in ether. It has a specific optical rotation of about +56°. Clopidogrel for oral administration is provided as either pink colored, round shaped, biconvex, de-bossed, film coated tablets containing 97.875 mg of clopidogrel bisulfate which is the molar equivalent of 75 mg of clopidogrel base or pink colored, modified oval shaped, de-bossed film coated tablets containing 391.5 mg of clopidogrel bisulfate which is the molar equivalent of 300 mg of clopidogrel base. Each tablet contains microcrystalline cellulose, mannitol, croscarmellose sodium, hydroxy propyl cellulose, hydroxy propyl methyl cellulose and hydrogenated castor oil as inactive ingredients. The film coating contains hypromellose, titanium dioxide, polyethylene glycol, red iron oxide and yellow iron oxide. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Clopidogrel tablets, USP 75 mg are available as pink colored, round shaped, biconvex, film coated tablets de-bossed on one side with SG and 124 on other side. Tablets are provided as follows: NDC 50228-124-30 Bottles of 30 NDC 50228-124-90 Bottles of 90 NDC 50228-124-05 Bottles of 500 NDC 50228-124-10 Bottles of 1,000 Clopidogrel tablets, USP 300 mg are available as pink colored, modified oval shaped, film coated tablets de-bossed on one side with SG and 121 on other side. Tablets are provided as follows: NDC 50228-121-30 Bottles of 30 NDC 50228-121-90 Bottles of 90 NDC 50228-121-05 Bottles of 500 Store at 25° C (77° F); excursions permitted to 15° to 30° C (59° to 86° F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Clopidogrel is a P2Y 12 platelet inhibitor indicated for: Acute coronary syndrome For patients with non–ST-segment elevation ACS (unstable angina [UA]/non-ST-elevation myocardial infarction [NSTEMI]), clopidogrel has been shown to reduce the rate of myocardial infarction (MI) and stroke. ( 1.1 ) For patients with ST-elevation myocardial infarction (STEMI), clopidogrel has been shown to reduce the rate of MI and stroke. ( 1.1 ) Recent MI, recent stroke, or established peripheral arterial disease. Clopidogrel has been shown to reduce the rate of MI and stroke. ( 1.2 ) 1.1 Acute Coronary Syndrome (ACS) • Clopidogrel is indicated to reduce the rate of myocardial infarction (MI) and stroke in patients with non–ST-segment elevation ACS (unstable angina [UA]/ non–ST -elevation myocardial infarction [NSTEMI]), including patients who are to be managed medically and those who are to be managed with coronary revascularization. Clopidogrel should be administered in conjunction with aspirin. • Clopidogrel is indicated to reduce the rate of myocardial infarction and stroke in patients with acute ST-elevation myocardial infarction (STEMI) who are to be managed medically. Clopidogrel should be administered in conjunction with aspirin. 1.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease In patients with established peripheral arterial disease or with a history of recent myocardial infarction (MI) or recent stroke clopidogrel is indicated to reduce the rate of MI and stroke.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Acute coronary syndrome ( 2.1 ) Initiate clopidogrel with a single 300-mg oral loading dose and then continue at 75 mg once daily. Initiate clopidogrel without a loading dose will delay establishment of an antiplatelet effect by several days. Recent MI, recent stroke, or established peripheral arterial disease: 75 mg once daily orally without a loading dose ( 2.2 ) 2.1 Acute Coronary Syndrome In patients who need an antiplatelet effect within hours, initiate clopidogrel with a single 300-mg oral loading dose and then continue at 75 mg once daily. Initiating clopidogrel without a loading dose will delay establishment of an antiplatelet effect by several days [see Clinical Pharmacology (12.3) and Clinical Studies (14.1) ] . 2.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease 75 mg once daily orally without a loading dose [see Clinical Pharmacology (12.3) and Clinical Studies (14.2) ] .
