Drug Catalog - Product Detail
DANTROLENE SODIUM CP 50MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-4422-01 | AMNEAL PHARMACEUTICALS | 100 | 50MG | CAPSULE |
PACKAGE FILES



Generic Name
DANTROLENE SODIUM
Substance Name
DANTROLENE SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA076856
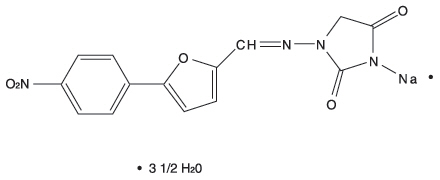
Description
DESCRIPTION The chemical formula of dantrolene sodium is hydrated 1-[[[5-(4-nitrophenyl)-2-furanyl]methylene] amino]-2, 4-imidazolidinedione sodium salt. It is an orange powder, slightly soluble in water, but due to its slightly acidic nature the solubility increases somewhat in alkaline solution. The anhydrous salt has a molecular weight of 336. The hydrated salt contains approximately 15% water (3-1/2 moles) and has a molecular weight of 399. The structural formula for the hydrated salt is: Dantrolene sodium, USP is supplied in capsules of 25 mg, 50 mg, and 100 mg. Inactive Ingredients: Each capsule contains croscarmellose sodium, gelatin, lactose monohydrate, magnesium stearate, pharmaceutical ink, pregelatinized starch, titanium dioxide, and yellow iron oxide. In addition, the 25 mg capsule contains D&C Yellow #10 and FD&C Green #3, the 50 mg capsule contains FD&C Blue #1, and the 100 mg capsule contains FD&C Red #40 and FD&C Yellow #6. Chemical Structure
How Supplied
HOW SUPPLIED Dantrolene Sodium Capsules USP, 25 mg are rich yellow opaque bodies and light green opaque caps. Each cap and body imprinted in black with G441. They are available as follows: Bottles of 100: NDC 0115-4411-01 Bottles of 500: NDC 0115-4411-02 Bottles of 1000: NDC 0115-4411-03 Dantrolene Sodium Capsules USP, 50 mg are rich yellow opaque bodies and light blue opaque caps. Each cap and body imprinted in black with G442. They are available as follows: Bottles of 100: NDC 0115-4422-01 Bottles of 500: NDC 0115-4422-02 Bottles of 1000: NDC 0115-4422-03 Dantrolene Sodium Capsules USP, 100 mg are rich yellow opaque bodies and reddish orange opaque caps. Each cap and body imprinted in black with G443. They are available as follows: Bottles of 100: NDC 0115-4433-01 Bottles of 500: NDC 0115-4433-02 Bottles of 1000: NDC 0115-4433-03 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture and humidity. Dispense in a tightly-closed, light-resistant container (USP).
Indications & Usage
INDICATIONS AND USAGE In Chronic Spasticity Dantrolene sodium capsules are indicated in controlling the manifestations of clinical spasticity resulting from upper motor neuron disorders (e.g., spinal cord injury, stroke, cerebral palsy, or multiple sclerosis). It is of particular benefit to the patient whose functional rehabilitation has been retarded by the sequelae of spasticity. Such patients must have presumably reversible spasticity where relief of spasticity will aid in restoring residual function. Dantrolene sodium capsules are not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders. If improvement occurs, it will ordinarily occur within the dosage titration (see DOSAGE AND ADMINISTRATION ), and will be manifested by a decrease in the severity of spasticity and the ability to resume a daily function not quite attainable without dantrolene sodium capsules. Occasionally, subtle but meaningful improvement in spasticity may occur with dantrolene sodium capsule therapy. In such instances, information regarding improvement should be solicited from the patient and those who are in constant daily contact and attendance with him. Brief withdrawal of dantrolene sodium capsules for a period of 2 to 4 days will frequently demonstrate exacerbation of the manifestations of spasticity and may serve to confirm a clinical impression. A decision to continue the administration of dantrolene sodium capsules on a long-term basis is justified if introduction of the drug into the patient's regimen: produces a significant reduction in painful and/or disabling spasticity such as clonus, or permits a significant reduction in the intensity and/or degree of nursing care required, or rids the patient of any annoying manifestation of spasticity considered important by the patient himself. In Malignant Hyperthermia Oral dantrolene sodium capsules are also indicated preoperatively to prevent or attenuate the development of signs of malignant hyperthermia in known, or strongly suspect, malignant hyperthermia susceptible patients who require anesthesia and/or surgery. Currently accepted clinical practices in the management of such patients must still be adhered to (careful monitoring for early signs of malignant hyperthermia, minimizing exposure to triggering mechanisms and prompt use of intravenous dantrolene sodium and indicated supportive measures should signs of malignant hyperthermia appear); see also the package insert for intravenous dantrolene sodium. Oral dantrolene sodium capsules should be administered following a malignant hyperthermic crisis to prevent recurrence of the signs of malignant hyperthermia.
Dosage and Administration
DOSAGE AND ADMINISTRATION For Use in Chronic Spasticity Prior to the administration of dantrolene sodium capsules, consideration should be given to the potential response to treatment. A decrease in spasticity sufficient to allow a daily function not otherwise attainable should be the therapeutic goal of treatment with dantrolene sodium capsules. Refer to INDICATIONS AND USAGE section for description of response to be anticipated. It is important to establish a therapeutic goal (regain and maintain a specific function such as therapeutic exercise program, utilization of braces, transfer maneuvers, etc.) before beginning dantrolene sodium capsule therapy. Dosage should be increased until the maximum performance compatible with the dysfunction due to underlying disease is achieved. No further increase in dosage is then indicated. Usual Dosage It is important that the dosage be titrated and individualized for maximum effect. The lowest dose compatible with optimal response is recommended. In view of the potential for liver damage in long-term dantrolene sodium capsule use, therapy should be stopped if benefits are not evident within 45 days. Adults The following gradual titration schedule is suggested. Some patients will not respond until higher daily dosage is achieved. Each dosage level should be maintained for seven days to determine the patient's response. If no further benefit is observed at the next higher dose, dosage should be decreased to the previous lower dose. 25 mg once daily for seven days, then 25 mg t.i.d. for seven days 50 mg t.i.d. for seven days 100 mg t.i.d. Therapy with a dose four times daily may be necessary for some individuals. Doses higher than 100 mg four times daily should not be used (see Box Warning ). Pediatric Patients The following gradual titration schedule is suggested. Some patients will not respond until higher daily dosage is achieved. Each dosage level should be maintained for seven days to determine the patient's response. If no further benefit is observed at the next higher dose, dosage should be decreased to the previous lower dose. 0.5 mg/kg once daily for seven days, then 0.5 mg/kg t.i.d. for seven days 1 mg/kg t.i.d. for seven days 2 mg/kg t.i.d. Therapy with a dose four times daily may be necessary for some individuals. Doses higher than 100 mg four times daily should not be used (see Box Warning ). For Malignant Hyperthermia Preoperatively: Administer 4 mg/kg/day to 8 mg/kg/day of oral dantrolene sodium caspules in 3 or 4 divided doses for one or two days prior to surgery, with the last dose being given approximately 3 to 4 hours before scheduled surgery with a minimum of water. This dosage will usually be associated with skeletal muscle weakness and sedation (sleepiness or drowsiness); adjustment can usually be made within the recommended dosage range to avoid incapacitation or excessive gastrointestinal irritation (including nausea and/or vomiting). Post Crisis Follow-up: Oral dantrolene sodium capsules should also be administered following a malignant hyperthermia crisis, in doses of 4 mg/kg per day to 8 mg/kg per day in four divided doses, for a one to three day period to prevent recurrence of the manifestations of malignant hyperthermia.
