Drug Catalog - Product Detail
DESIPRAMINE HCL TB 75MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45963-0344-02 | ACTAVIS | 100 | 75MG | TABLET |
PACKAGE FILES


Generic Name
DESIPRAMINE HYDROCHLORIDE
Substance Name
DESIPRAMINE HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA074430
Description
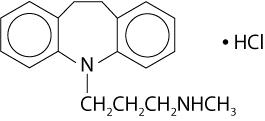
DESCRIPTION Desipramine hydrochloride, USP is an antidepressant drug of the tricyclic type, and is chemically: 5 H -Dibenz[ b,f ]azepine-5-propanamine,10,11-dihydro- N -methyl-, monohydrochloride. Inactive Ingredients The following inactive ingredients are contained in all dosage strengths: croscarmellose sodium, hypromellose, anhydrous lactose, magnesium stearate, polyethylene glycol, polysorbate 80, povidone, sodium lauryl sulfate and titanium dioxide. The 25 mg, 50 mg, 75 mg, and 100 mg tablets also contain FD&C Blue No. 1 Aluminum Lake. 9d202fae-figure-01
How Supplied
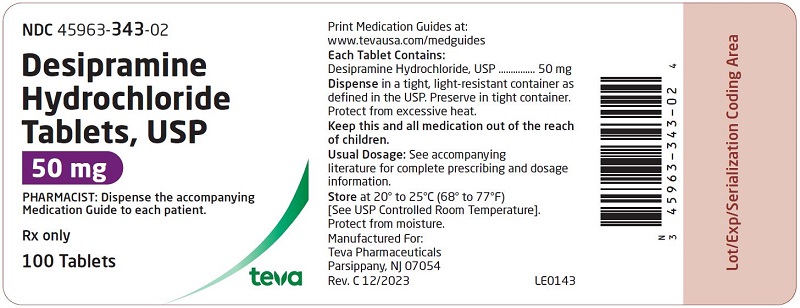
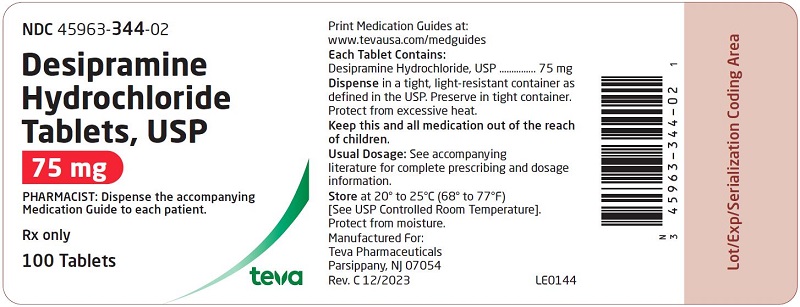
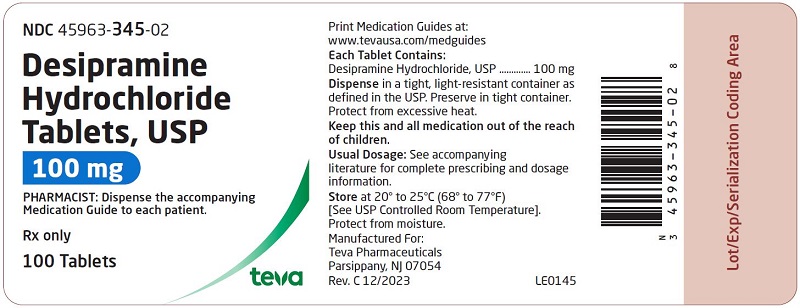
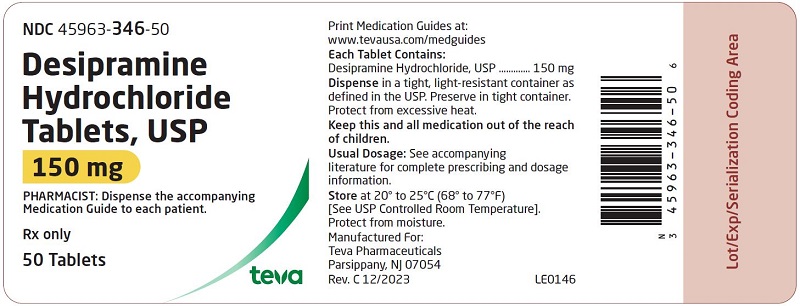
HOW SUPPLIED: Desipramine hydrochloride tablets, USP are supplied as follows: 10 mg – Each white to off-white, round, unscored, biconvex with flat edge, film-coated tablet, debossed with over 341 on one side and plain on the other side. Tablets are supplied in bottles of 100 (NDC 45963-341-02) with a non-child resistant closure. 25 mg – Each light blue, round, unscored, biconvex with flat edge, film-coated tablet, debossed with over 342 on one side and plain on the other side. Tablets are supplied in bottles of 100 (NDC 45963-342-02) with a non-child resistant closure. 50 mg - Each blue, round, unscored, biconvex with flat edge, film-coated tablet, debossed with over 343 on one side and plain on the other side. Tablets are supplied in bottles of 100 (NDC 45963-343-02) with a non-child resistant closure. 75 mg – Each light blue, round, unscored, biconvex with flat edge, film-coated tablet, debossed with over 344 on one side and plain on the other side. Tablets are supplied in bottles of 100 (NDC 45963-344-02) with a non-child resistant closure. 100 mg – Each blue, round, unscored, biconvex with flat edge, film-coated tablet, debossed with over 345 on one side and plain on the other side. Tablets are supplied in bottles of 100 (NDC 45963-345-02) with a non-child resistant closure. 150 mg – Each white to off-white, round, unscored, biconvex with flat edge, film-coated tablet, debossed with over 346 on one side and plain on the other side. Tablets are supplied in bottles of 50 (NDC 45963-346-50) with a non-child resistant closure. Dispense in a tight, light-resistant container as defined in the USP. Preserve in tight container. Protect from excessive heat. Storage: Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from moisture. Dispense with Medication Guide available at: www.tevausa.com/medguides Manufactured For: Teva Pharmaceuticals Parsippany, NJ 07054 Rev. C 9/2024 9d202fae-figure-02 9d202fae-figure-03 9d202fae-figure-04 9d202fae-figure-05 9d202fae-figure-06 9d202fae-figure-07
Indications & Usage
INDICATIONS AND USAGE Desipramine hydrochloride tablets are indicated for the treatment of depression.
Dosage and Administration
DOSAGE AND ADMINISTRATION Not recommended for use in children (see WARNINGS ). Lower dosages are recommended for elderly patients and adolescents. Lower dosages are also recommended for outpatients compared to hospitalized patients, who are closely supervised. Dosage should be initiated at a low level and increased according to clinical response and any evidence of intolerance. Following remission, maintenance medication may be required for a period of time and should be at the lowest dose that will maintain remission. Usual Adult Dose The usual adult dose is 100 mg to 200 mg per day. In more severely ill patients, dosage may be further increased gradually to 300 mg/day if necessary. Dosages above 300 mg/day are not recommended. Dosage should be initiated at a lower level and increased according to tolerance and clinical response. Treatment of patients requiring as much as 300 mg should generally be initiated in hospitals, where regular visits by the physician, skilled nursing care, and frequent electrocardiograms (ECGs) are available. The best available evidence of impending toxicity from very high doses of desipramine is prolongation of the QRS or QT intervals on the ECG. Prolongation of the PR interval is also significant, but less closely correlated with plasma levels. Clinical symptoms of intolerance, especially drowsiness, dizziness, and postural hypotension, should also alert the physician to the need for reduction in dosage. Initial therapy may be administered in divided doses or a single daily dose. Maintenance therapy may be given on a once-daily schedule for patient convenience and compliance. Adolescent and Geriatric Dose The usual adolescent and geriatric dose is 25 mg to 100 mg daily. Dosage should be initiated at a lower level and increased according to tolerance and clinical response to a usual maximum of 100 mg daily. In more severely ill patients, dosage may be further increased to 150 mg/day. Doses above 150 mg/day are not recommended in these age groups. Initial therapy may be administered in divided doses or a single daily dose. Maintenance therapy may be given on a once-daily schedule for patient convenience and compliance. Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders : At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with desipramine hydrochloride. Conversely, at least 14 days should be allowed after stopping desipramine hydrochloride before starting an MAOI intended to treat psychiatric disorders (see CONTRAINDICATIONS ). Use of Desipramine Hydrochloride With Other MAOI’s Such as Linezolid or Methylene Blue: Do not start desipramine hydrochloride in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see CONTRAINDICATIONS ). In some cases, a patient already receiving desipramine hydrochloride therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, desipramine hydrochloride should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with desipramine hydrochloride may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see WARNINGS ). The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with desipramine hydrochloride is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see WARNINGS ).
