Drug Catalog - Product Detail
DESMOPRESSIN ACETATE 0.2MG TB 100CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
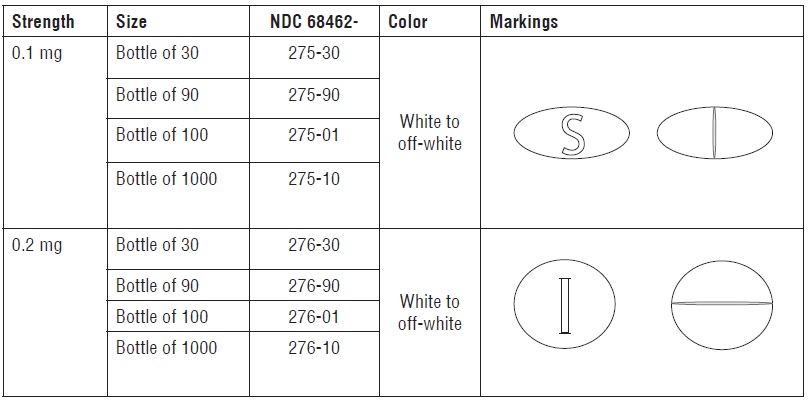
| 68462-0276-01 | GLENMARK PHARMACEUTICALS | 100 | 0.2MG | TABLET |
PACKAGE FILES



Generic Name
DESMOPRESSIN ACETATE
Substance Name
DESMOPRESSIN ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA201831
Description
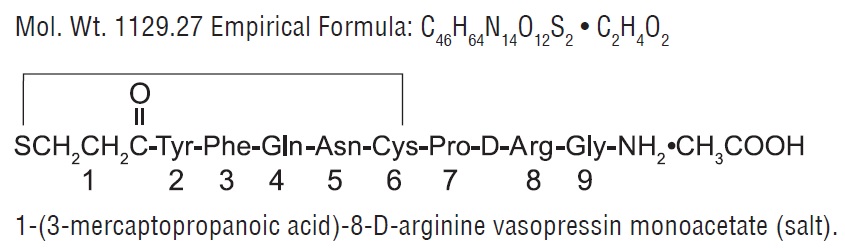
DESCRIPTION Desmopressin Acetate Tablets are a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows: Desmopressin acetate tablets, for oral administration, contain either 0.1 or 0.2 mg desmopressin acetate, USP. Inactive ingredients include: lactose monohydrate, potato starch, talc, magnesium stearate and povidone. structure
How Supplied
HOW SUPPLIED Desmopressin Acetate Tablets 0.1mg : White to off-white, oval, biconvex, uncoated tablets debossed ‘S’ on one side and scored on the other side. Desmopressin Acetate Tablets 0.2 mg : White to off-white, round, biconvex, uncoated tablets debossed ‘I’ on one side and scored on the other side. Store at 20° to 25°C (68 to 77°F) [see USP Controlled Room Temperature]. Avoid exposure to excessive heat or light. This product should be dispensed in a container with a child-resistant cap. Keep out of the reach of children. Manufactured by: Glenmark Pharmaceuticals Ltd. Colvale-Bardez, Goa 403 513, India Manufactured for: Glenmark Pharmaceuticals Inc., USA Mahwah, NJ 07430 Questions? 1 (888)721-7115 www.glenmarkpharma.com/usa March 2015 how-supplied logo
Indications & Usage
INDICATIONS AND USAGE Central Diabetes Insipidus: Desmopressin acetate tablets are indicated as antidiuretic replacement therapy in the management of central diabetes insipidus and for the management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. Desmopressin acetate tablets are ineffective for the treatment of nephrogenic diabetes insipidus. Patients were selected for therapy based on the diagnosis by means of the water deprivation test, the hypertonic saline infusion test, and/or response to antidiuretic hormone. Continued response to desmopressin acetate can be monitored by measuring urine volume and osmolality. Primary Nocturnal Enuresis: Desmopressin acetate tablets are indicated for the management of primary nocturnal enuresis. Desmopressin acetate tablets may be used alone or as an adjunct to behavioral conditioning or other non-pharmacologic intervention.
Dosage and Administration
DOSAGE AND ADMINISTRATION Central Diabetes Insipidus: The dosage of desmopressin acetate tablets must be determined for each individual patient and adjusted according to the diurnal pattern of response. Response should be estimated by two parameters: adequate duration of sleep and adequate, not excessive, water turnover. Patients previously on intranasal desmopressin acetate therapy should begin tablet therapy twelve hours after the last intranasal dose. During the initial dose titration period, patients should be observed closely and appropriate safety parameters measured to assure adequate response. Patients should be monitored at regular intervals during the course of desmopressin acetate tablets therapy to assure adequate antidiuretic response. Modifications in dosage regimen should be implemented as necessary to assure adequate water turnover. Fluid restriction should be observed. (See WARNINGS , PRECAUTIONS , Pediatric Use and Geriatric Use.) Adults and Children: It is recommended that patients be started on doses of 0.05 mg (1/2 of the 0.1 mg tablet) two times a day and individually adjusted to their optimum therapeutic dose. Most patients in clinical trials found that the optimal dosage range is 0.1 mg to 0.8 mg daily, administered in divided doses. Each dose should be separately adjusted for an adequate diurnal rhythm of water turnover. Total daily dosage should be increased or decreased in the range of 0.1 mg to 1.2 mg divided into two or three daily doses as needed to obtain adequate antidiuresis. See Pediatric Use subsection for special considerations when administering desmopressin acetate to pediatric diabetes insipidus patients. Geriatric Use: This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY , Human Pharmacokinetics, CONTRAINDICATIONS , and PRECAUTIONS , Geriatric Use.) Primary Nocturnal Enuresis: The dosage of desmopressin acetate tablets must be determined for each individual patient and adjusted according to response. Patients previously on intranasal desmopressin acetate therapy can begin tablet therapy the night following (24 hours after) the last intranasal dose. The recommended initial dose for patients age 6 years and older is 0.2 mg at bedtime. The dose may be titrated up to 0.6 mg to achieve the desired response. Fluid restriction should be observed, and fluid intake should be limited to a minimum from 1 hour before desmopressin administration, until the next morning, or at least 8 hours after administration. (See WARNINGS , PRECAUTIONS , Pediatric Use and Geriatric Use.)
