Drug Catalog - Product Detail
DESOGESTREL/ETHINYL ESTRADIOL (KARIVA) TB 0.15/0.02/0.01MG 6X28
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00555-9050-58 | TEVA PHARMACEUTICALS USA | 28 | 0.15-0.02/0.01MG (21/5) | TABLET |
PACKAGE FILES
Generic Name
DESOGESTREL/ETHINYL ESTRADIOL AND ETHINYL ESTRADIOL
Substance Name
Product Type
HUMAN PRESCRIPTION DRUG
Route
Application Number
ANDA075863
Description
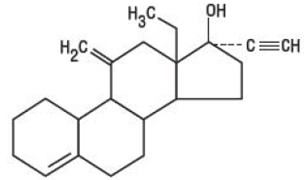
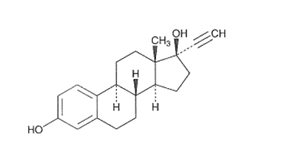
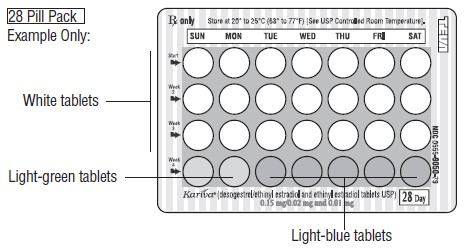
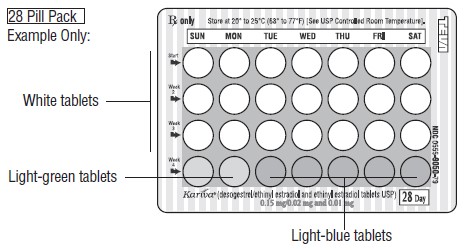
DESCRIPTION Kariva ® (desogestrel/ethinyl estradiol and ethinyl estradiol tablets USP) provides an oral contraceptive regimen of 21 white, round tablets each containing 0.15 mg desogestrel (13-ethyl-11-methylene-18,19-dinor-17 alpha-pregn-4-en-20-yn-17-ol), 0.02 mg ethinyl estradiol, USP (19-nor-17 alpha-pregna-1,3,5 (10)-trien-20-yne-3,17-diol), and inactive ingredients which include colloidal silicon dioxide, hypromellose, lactose monohydrate, polyethylene glycol, povidone, pregelatinized corn starch, stearic acid, and vitamin E, followed by 2 inert light-green, round tablets with the following inactive ingredients: FD&C blue no. 1 aluminum lake, FD&C yellow no. 6 aluminum lake, D&C yellow no. 10 aluminum lake, lactose monohydrate, magnesium stearate, microcrystalline cellulose and pregelatinized corn starch. Kariva ® also contains 5 light-blue, round tablets containing 0.01 mg ethinyl estradiol, USP (19-nor-17 alpha-pregna-1,3,5 (10)-trien-20-yne-3,17-diol) and inactive ingredients which include colloidal silicon dioxide, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, hypromellose, lactose monohydrate, polydextrose, polyethylene glycol, povidone, pregelatinized corn starch, stearic acid, titanium dioxide, triacetin and vitamin E. The structural formulas are as follows: DESOGESTREL C 22 H 30 O M.W. 310.48 ETHINYL ESTRADIOL, USP C 20 H 24 O 2 M.W. 296.40 The 21 white tablets meet USP Dissolution Test 2. 1 Ethinyl Estradiol, USP
How Supplied
HOW SUPPLIED Kariva ® (desogestrel/ethinyl estradiol and ethinyl estradiol tablets USP) contains 21 round, white, film-coated, biconvex tablets, 2 round, light-green tablets and 5 round, light-blue, film-coated, biconvex tablets in a blister card. Each white tablet (debossed with “ dp ” on one side and “ 021 ” on the other side) contains 0.15 mg desogestrel and 0.02 mg ethinyl estradiol, USP. Each light-green tablet (debossed with “ dp ” on one side and “ 331 ” on the other side) contains inert ingredients. Each light-blue tablet (debossed with “ dp ” on one side and “ 022 ” on the other side) contains 0.01 mg ethinyl estradiol, USP. Box of 6 Blister Cards (NDC: 0555-9050-58) Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Keep this and all medications out of the reach of children.
Indications & Usage
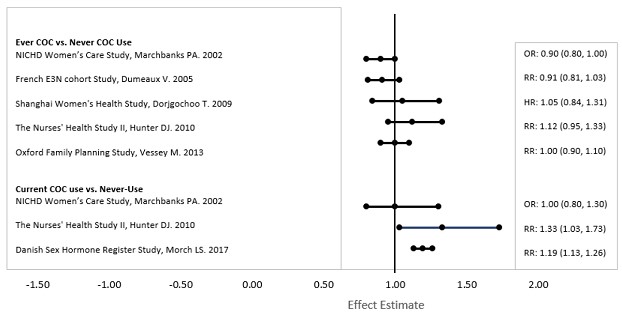
INDICATIONS AND USAGE Kariva ® (desogestrel/ethinyl estradiol and ethinyl estradiol tablets USP) is indicated for the prevention of pregnancy in women who elect to use this product as a method of contraception. Oral contraceptives are highly effective. Table II lists the typical accidental pregnancy rates for users of combination oral contraceptives and other methods of contraception. The efficacy of these contraceptive methods, except sterilization, depends upon the reliability with which they are used. Correct and consistent use of these methods can result in lower failure rates. TABLE II: Percentage of women experiencing an unintended pregnancy during the first year of typical use and the first year of perfect use of contraception and the percentage continuing use at the end of the first year, United States. % of Women Experiencing an Unintended Pregnancy within the First Year of Use % of Women Continuing Use at One Year a (4) Method (1) Typical Use b (2) Perfect Use c (3) Chance d 85 85 Spermicides e 26 6 40 Periodic abstinence 25 63 Calendar 9 Ovulation Method 3 Sympto-Thermal f 2 Post-Ovulation 1 Withdrawal 19 4 Cap g Parous Women 40 26 42 Nulliparous Women 20 9 56 Sponge Parous Women 40 20 42 Nulliparous Women 20 9 56 Diaphragm g 20 6 56 Condom h Female (Reality) 21 5 56 Male 14 3 61 Pill 5 71 Progestin Only 0.5 Combined 0.1 IUD Progesterone T 2.0 1.5 81 Copper T 380A 0.8 0.6 78 LNg 20 0.1 0.1 81 Depo-Provera 0.3 0.3 70 Norplant and Norplant-2 0.05 0.05 88 Female sterilization 0.5 0.5 100 Male sterilization 0.15 0.10 100 Adapted from Hatcher et al., 1998, ref #1. a) Among couples attempting to avoid pregnancy, the percentage who continue to use a method for one year. b) Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. c) Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. d) The percents becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 85%) to represent the percent who would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether. e) Foams, creams, gels, vaginal suppositories, and vaginal film. f) Cervical mucus (ovulation) method supplemented by calendar in the pre-ovulatory and basal body temperature in the post-ovulatory phases. g) With spermicidal cream or jelly. h) Without spermicides.
Dosage and Administration
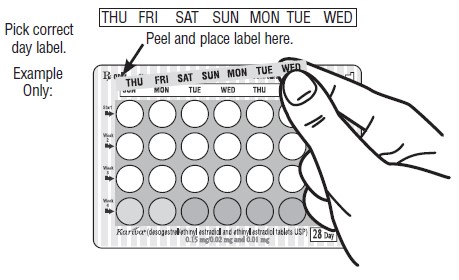
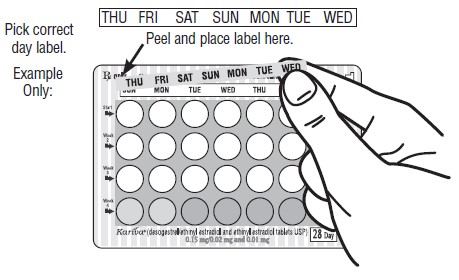
DOSAGE AND ADMINISTRATION To achieve maximum contraceptive effectiveness, Kariva ® must be taken exactly as directed and at intervals not exceeding 24 hours. Kariva ® may be initiated using either a Sunday start or a Day 1 start. NOTE: Each cycle pack dispenser is preprinted with the days of the week, starting with Sunday, to facilitate a Sunday start regimen. Six different “day label strips” are provided with each cycle pack dispenser in order to accommodate a Day 1 start regimen. In this case, the patient should place the self-adhesive “day label strip” that corresponds to her starting day over the preprinted days. IMPORTANT: The possibility of ovulation and conception prior to initiation of use of Kariva ® should be considered. The use of Kariva ® for contraception may be initiated 4 weeks postpartum in women who elect not to breastfeed. When the tablets are administered during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered (see CONTRAINDICATIONS and WARNINGS concerning thromboembolic disease. See also PRECAUTIONS for Nursing mothers ). If the patient starts on Kariva ® postpartum, and has not yet had a period, she should be instructed to use another method of contraception until a white tablet has been taken daily for 7 days. SUNDAY START When initiating a Sunday start regimen, another method of contraception should be used until after the first 7 consecutive days of administration. Using a Sunday start, tablets are taken daily without interruption as follows: The first white tablet should be taken on the first Sunday after menstruation begins (if menstruation begins on Sunday, the first white tablet is taken on that day). One white tablet is taken daily for 21 days, followed by 1 light-green (inert) tablet daily for 2 days and 1 light-blue (active) tablet daily for 5 days. For all subsequent cycles, the patient then begins a new 28-tablet regimen on the next day (Sunday) after taking the last light-blue tablet. [If switching from a Sunday start oral contraceptive, the first Kariva ® (desogestrel/ethinyl estradiol and ethinyl estradiol) tablet should be taken on the second Sunday after the last tablet of a 21 day regimen or should be taken on the first Sunday after the last inactive tablet of a 28 day regimen.] If a patient misses 1 white tablet, she should take the missed tablet as soon as she remembers. If the patient misses 2 consecutive white tablets in Week 1 or Week 2, the patient should take 2 tablets the day she remembers and 2 tablets the next day; thereafter, the patient should resume taking 1 tablet daily until she finishes the cycle pack. The patient should be instructed to use a back-up method of birth control if she has intercourse in the 7 days after missing pills. If the patient misses 2 consecutive white tablets in the third week or misses 3 or more white tablets in a row at any time during the cycle, the patient should keep taking 1 white tablet daily until the next Sunday. On Sunday the patient should throw out the rest of that cycle pack and start a new cycle pack that same day. The patient should be instructed to use a back-up method of birth control if she has intercourse in the 7 days after missing pills. DAY 1 START Counting the first day of menstruation as “Day 1”, tablets are taken without interruption as follows: One white tablet daily for 21 days, one light-green (inert) tablet daily for 2 days followed by 1 light-blue (ethinyl estradiol) tablet daily for 5 days. For all subsequent cycles, the patient then begins a new 28-tablet regimen on the next day after taking the last light-blue tablet. [If switching directly from another oral contraceptive, the first white tablet should be taken on the first day of menstruation which begins after the last ACTIVE tablet of the previous product.] If a patient misses 1 white tablet, she should take the missed tablet as soon as she remembers. If the patient misses 2 consecutive white tablets in Week 1 or Week 2, the patient should take 2 tablets the day she remembers and 2 tablets the next day; thereafter, the patient should resume taking 1 tablet daily until she finishes the cycle pack. The patient should be instructed to use a back-up method of birth control if she has intercourse in the 7 days after missing pills. If the patient misses 2 consecutive white tablets in the third week or if the patient misses 3 or more white tablets in a row at any time during the cycle, the patient should throw out the rest of that cycle pack and start a new cycle pack that same day. The patient should be instructed to use a back-up method of birth control if she has intercourse in the 7 days after missing pills.
