Drug Catalog - Product Detail
Dextroamphetamine Sulfate Cap ER 24HR 15 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00406-8962-01 | MALLINCKRODT PHARM | 100 | 15MG | CAPSULE |
PACKAGE FILES





Generic Name
DEXTROAMPHETAMINE SULFATE
Substance Name
DEXTROAMPHETAMINE SULFATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA076353
Description
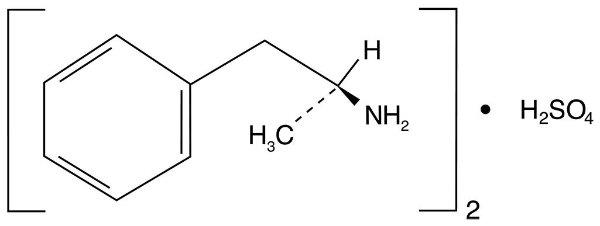
DESCRIPTION Dextroamphetamine sulfate extended-release capsule is the dextro isomer of the compound d,l -amphetamine sulfate, a sympathomimetic amine of the amphetamine group. Chemically, dextroamphetamine is d -alpha-methylphenethylamine, and is present in all forms of dextroamphetamine sulfate extended-release capsules as the neutral sulfate. Structural formula: Chemical Structure Dextroamphetamine Sulfate Extended-Release Capsules Each dextroamphetamine sulfate extended-release capsule is so prepared that an initial dose is released promptly and the remaining medication is released gradually over a prolonged period. Each capsule, with white opaque cap and white opaque body, contains dextroamphetamine sulfate USP. The 5 mg capsule is imprinted with an ® on the cap and is imprinted 8960 5 mg on the body in black. The 10 mg capsule is imprinted with an ® on the cap and is imprinted 8961 10 mg on the body in blue. The 15 mg capsule is imprinted with an ® on the cap and is imprinted 8962 15 mg on the body in pink. Inactive ingredients consist of sugar spheres, titanium dioxide, gelatin, shellac glaze-45%, SD-45 alcohol, iron oxide black, propylene glycol, FD&C Blue #2/Indigo Carmine Lake, FD&C Red #40/Allura Red AC Lake, FD&C Blue #1/Brilliant Blue FCF Lake, D&C Yellow #10 Lake, SD3A alcohol, shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, strong ammonia solution, FD&C Blue #2 Aluminum Lake, D&C Red #7 Calcium Lake, hydroxypropyl methylcellulose/hypromellose, macrogol/polyethylene glycol, purified water, ethylcellulose, ammonium hydroxide 28%, medium chain triglycerides, oleic acid. Boxed M Boxed M Boxed M
How Supplied
HOW SUPPLIED Dextroamphetamine Sulfate Extended-Release Capsules Each capsule, with white opaque cap and white opaque body, contains dextroamphetamine sulfate. The 5 mg capsule is imprinted with an ® on the cap and is imprinted 8960 5 mg on the body in black. The 10 mg capsule is imprinted with an ® on the cap and is imprinted 8961 10 mg on the body in blue. The 15 mg capsule is imprinted with an ® on the cap and is imprinted 8962 15 mg on the body in pink. 5 mg Bottles of 100……………….NDC 0406-8960-01 10 mg Bottles of 100……………….NDC 0406-8961-01 15 mg Bottles of 100……………….NDC 0406-8962-01 Store at controlled room temperature between 20° to 25°C (68° to 77°F) [see USP]. Dispense in a tight, light-resistant container with a child-resistant closure. Mallinckrodt, the “M” brand mark, the Mallinckrodt Pharmaceuticals logo and are trademarks of a Mallinckrodt company. © 2023 Mallinckrodt. SpecGx LLC Webster Groves, MO 63119 USA Rev 10/2023 Mallinckrodt™ Pharmaceuticals Boxed M Boxed M Boxed M Boxed M
Indications & Usage
INDICATIONS AND USAGE Dextroamphetamine sulfate extended-release capsules are indicated in: Narcolepsy Attention Deficit Disorder with Hyperactivity As an integral part of a total treatment program that typically includes other measures (psychological, educational, social) for patients (ages 6 years to 16 years) with this syndrome. A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV) implies the presence of the hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in 2 or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least 6 of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least 6 of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; “on the go”; excessive talking; blurting answers; can’t wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met. Special Diagnostic Considerations Specific etiology of this syndrome is unknown, and there is no single diagnostic test. Adequate diagnosis requires the use of medical and special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the patient and not solely on the presences of the required number of DSM-IV characteristics. Need for Comprehensive Treatment Program Dextroamphetamine sulfate extended-release capsules are indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all patients with this syndrome. Stimulants are not intended for use in patients who exhibit symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician’s assessment of the chronicity and severity of the patient’s symptoms.
Dosage and Administration
DOSAGE AND ADMINISTRATION Amphetamines should be administered at the lowest effective dosage and dosage should be individually adjusted. Late evening doses should be avoided because of the resulting insomnia. Prior to treating patients with dextroamphetamine sulfate extended-release capsules, assess: for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) ( see WARNINGS ). the family history and clinically evaluate patients for motor or verbal tics or Tourette’s syndrome ( see WARNINGS ). Narcolepsy Usual dose is 5 to 60 mg per day in divided doses, depending on the individual patient response. Narcolepsy seldom occurs in children under 12 years of age; however, when it does, dextroamphetamine sulfate extended-release capsules may be used. The suggested initial dose for patients aged 6 to 12 is 5 mg daily; daily dose may be raised in increments of 5 mg at weekly intervals until an optimal response is obtained. In patients 12 years of age and older, start with 10 mg daily; daily dosage may be raised in increments of 10 mg at weekly intervals until an optimal response is obtained. If bothersome adverse reactions appear (e.g., insomnia or anorexia), dosage should be reduced. Dextroamphetamine sulfate extended-release capsules may be used for once-a-day dosage wherever appropriate. Attention Deficit Disorder with Hyperactivity The dextroamphetamine sulfate extended-release capsule formulation is not recommended for pediatric patients younger than 6 years of age. In pediatric patients 6 years of age and older, start with 5 mg once or twice daily; daily dosage may be raised in increments of 5 mg at weekly intervals until optimal response is obtained. Only in rare cases will it be necessary to exceed a total of 40 mg per day. Dextroamphetamine sulfate extended-release capsules may be used for once-a-day dosage wherever appropriate.
