Drug Catalog - Product Detail
DILTIAZEM HCL ER (CD) CP 360MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 47335-0679-81 | SUN PHARMACEUTICALS | 90 | 360MG | CAPSULE |
PACKAGE FILES


Generic Name
DILTIAZEM HYDROCHLORIDE
Substance Name
DILTIAZEM HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090492
Description
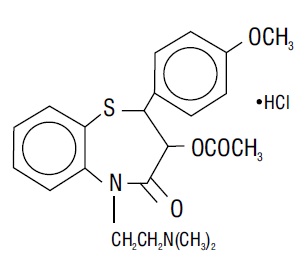
DESCRIPTION Diltiazem hydrochloride is a calcium ion cellular influx inhibitor (slow channel blocker or calcium antagonist). Chemically, diltiazem hydrochloride is 1,5-benzothiazepin-4(5H)one, 3-(acetyloxy)-5-[2-(dimethylamino) ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride,(+)-cis-. The chemical structure is: Diltiazem hydrochloride is a white to off-white crystalline powder with a bitter taste. It is soluble in water, formic acid, methanol, and chloroform. It has a molecular weight of 450.98. Diltiazem hydrochloride extended-release capsule, USP (Once-a-day dosage) is formulated as a once-a-day extended-release capsule containing 120 mg diltiazem hydrochloride (equivalent to 110.3 mg diltiazem), 180 mg diltiazem hydrochloride (equivalent to 165.45 mg diltiazem), 240 mg diltiazem hydrochloride (equivalent to 220.6 mg diltiazem), 300 mg diltiazem hydrochloride (equivalent to 275.75 mg diltiazem), or 360 mg diltiazem hydrochloride (equivalent to 330.9 mg diltiazem). The 120 mg, 180 mg, 240 mg, 300 mg and 360 mg contain: sugar spheres, hypromellose, talc, ethylcellulose, triethyl citrate, ammonio methacrylate copolymer dispersion type B, acetyl tributyl citrate, polysorbate 80, and magnesium stearate. The capsule shells contain gelatin, sodium lauryl sulfate, titanium dioxide, FD & C Blue 1 and iron oxide black (180 mg, 240 mg, 300 mg and 360 mg). Imprinting ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water. For oral administration. FDA approved dissolution test specifications differ from USP. structure
How Supplied
HOW SUPPLIED Diltiazem Hydrochloride Extended-Release Capsules, USP (Once-a-Day Dosage) Strength Quantity NDC Number Description 120 mg Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC 47335-675-83 47335-675-81 47335-675-19 47335-675-13 47335-675-18 Hard gelatin capsules, size ‘2’Light turquoise blue colored cap and body, with “675” imprinted in black ink on cap and body, containing white to off white pellets. 180 mg Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC 47335-676-83 47335-676-81 47335-676-19 47335-676-13 47335-676-18 Hard gelatin capsules, size ‘0’ blue colored cap and light turquoise blue body, with “676” imprinted in black ink on cap and body, containing white to off white pellets. 240 mg Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC 47335-677-83 47335-677-81 47335-677-19 47335-677-13 47335-677-18 Hard gelatin capsules, size ‘0’ blue colored cap and blue colored body, with “677” imprinted in black ink on cap and body, containing white to off white pellets. 300 mg Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC 47335-678-83 47335-678-81 47335-678-19 47335-678-13 47335-678-18 Hard gelatin capsules, size ‘00’ blue colored cap and gray colored body, with “678” imprinted in black ink on cap and body, containing white to off white pellets. 360 mg Bottle of 30 CRC Bottle of 90 CRC Bottle of 90 NCRC Bottle of 500 NCRC Bottle of 1000 NCRC 47335-679-83 47335-679-81 47335-679-19 47335-679-13 47335-679-18 Hard gelatin capsules, size ‘00’ blue colored cap and white colored body with “679” imprinted in black ink on cap and body, containing white to off white pellets. Storage Condition Store at 20° to 25°C (68° to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Avoid excessive humidity. Dispense in light-resistant, tight container with child-resistant closure.
Indications & Usage
INDICATIONS AND USAGE Diltiazem hydrochloride extended-release capsules (Once-a-day dosage) are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive medications. Diltiazem hydrochloride extended-release capsules (Once-a-day dosage) are indicated for the management of chronic stable angina and angina due to coronary artery spasm.
Dosage and Administration
DOSAGE AND ADMINISTRATION Patients controlled on diltiazem alone or in combination with other medications may be switched to diltiazem hydrochloride extended-release capsules at the nearest equivalent total daily dose. Higher doses of diltiazem hydrochloride extended-release capsules may be needed in some patients. Monitor patients closely. Subsequent titration to higher or lower doses may be necessary. There is limited general clinical experience with doses above 360 mg, but doses to 540 mg have been studied in clinical trials. The incidence of side effects increases as the dose increases with first-degree AV block, dizziness, and sinus bradycardia bearing the strongest relationship to dose. Hypertension: Adjust dosage to individual patient needs. When used as monotherapy, reasonable starting doses are 180 to 240 mg once daily, although some patients may respond to lower doses. Maximum antihypertensive effect is usually observed by 14 days of chronic therapy; therefore, schedule dosage adjustments accordingly. The usual dosage range studied in clinical trials was 240 to 360 mg once daily. Individual patients may respond to higher doses of up to 480 mg once daily. Angina: Dosages for the treatment of angina should be adjusted to each patient's needs, starting with a dose of 120 or 180 mg once daily. Individual patients may respond to higher doses of up to 480 mg once daily. When necessary, titration may be carried out over a 7- to 14-day period. Concomitant Use with Other Cardiovascular Agents: Sublingual NTG: May be taken as required to abort acute anginal attacks during diltiazem hydrochloride therapy. Prophylactic Nitrate Therapy: Diltiazem hydrochloride extended-release capsules may be safely coadministered with short- and long-acting nitrates. Beta-blockers : (see WARNINGS and PRECAUTIONS ). Antihypertensives: Diltiazem hydrochloride extended-release capsules have an additive antihypertensive effect when used with other antihypertensive agents. Therefore, the dosage of diltiazem hydrochloride extended-release capsules or the concomitant antihypertensives may need to be adjusted when adding one to the other.
