Drug Catalog - Product Detail
DIMERCAPROL FOR INJECTION, USP (BAL IN OIL) INJECT. 100MG/ML 10X3ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 17478-0526-03 | AKORN | 3 | 100MG/ML | SOLUTION |
PACKAGE FILES


Generic Name
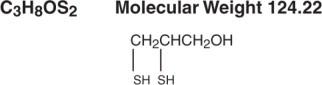
Substance Name
Product Type
Route
Application Number
Description
DESCRIPTION Dimercaprol Injection USP is a colorless or almost colorless liquid chelating agent having a disagreeable, mercaptan-like odor. Each 1 mL sterile BAL in Oil (Dimercaprol Injection USP) contains: 100 mg Dimercaprol in 200 mg Benzyl Benzoate and 700 mg Peanut Oil.
How Supplied
HOW SUPPLIED 3 mL (100 mg/mL) ampules, box of 10 (NDC 17478-526-03). STORAGE: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
INDICATIONS BAL in Oil (Dimercaprol Injection USP) is indicated in the treatment of arsenic, gold and mercury poisoning. It is indicated in acute lead poisoning when used concomitantly with Edetate Calcium Disodium Injection USP. Dimercaprol Injection USP is effective for use in acute poisoning by mercury salts if therapy is begun within one or two hours following ingestion. It is not very effective for chronic mercury poisoning. Dimercaprol Injection USP is of questionable value in poisoning caused by other heavy metals such as antimony and bismuth. It should not be used in iron, cadmium, or selenium poisoning because the resulting dimercaprol-metal complexes are more toxic than the metal alone, especially to the kidneys.
Dosage and Administration
DOSAGE AND ADMINISTRATION By deep intramuscular injection only. For mild arsenic or gold poisoning, 2.5 mg/kg of body weight four times daily for two days, two times on the third day, and once daily thereafter for ten days; for severe arsenic or gold poisoning, 3 mg/kg every four hours for two-days, four times on the third day, then twice daily thereafter for ten days. For mercury poisoning, 5 mg/kg initially, followed by 2.5 mg/kg one or two times daily for ten days. For acute lead encephalopathy, 4 mg/kg body weight is given alone in the first dose and thereafter at four-hour intervals in combination with Edetate Calcium Disodium Injection USP administered at a separate site. For less severe poisoning the dose can be reduced to 3 mg/kg after the first dose. Treatment is maintained for two to seven days depending on clinical response. Successful treatment depends on beginning injections at the earliest possible moment and on the use of adequate amounts at frequent intervals. Other supportive measures should always be used in conjunction with BAL in Oil (Dimercaprol Injection USP) therapy. BAL in Oil should be inspected visually for particulate matter and discoloration prior to administration.
