Drug Catalog - Product Detail
DIVALPROEX SODIUM ER 500MG TAB 500CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68180-0261-02 | LUPIN PHARMACEUTICALS | 500 | 500MG | NA |
PACKAGE FILES

Generic Name
DIVALPROEX SODIUM
Substance Name
DIVALPROEX SODIUM
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA209286
Description
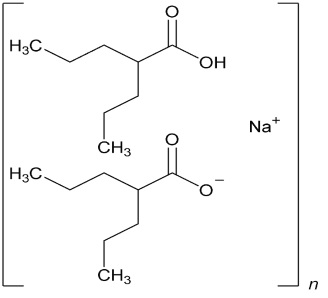
11 DESCRIPTION Divalproex sodium is a stable co-ordination compound comprised of sodium valproate and valproic acid in a 1:1 molar relationship and formed during the partial neutralization of valproic acid with 0.5 equivalent of sodium hydroxide. Chemically it is designated as sodium hydrogen bis(2-propylpentanoate). Divalproex sodium has the following structure: Image 1 Divalproex sodium occurs as a white to off white powder. Divalproex sodium extended-release 250 mg and 500 mg tablets are for oral administration. Divalproex sodium extended-release tablets, USP contain divalproex sodium in a once-a-day extended-release formulation equivalent to 250 mg and 500 mg of valproic acid. Inactive Ingredients Divalproex Sodium Extended-Release Tablets USP, 250 mg and 500 mg: ethyl acrylate and methyl methacrylate copolymer dispersion, ethyl cellulose, hypromellose, iron oxide black (ferrosoferric oxide), lactose monohydrate, magnesium stearate, microcrystalline cellulose, propylene glycol, polyethylene glycol, polyvinyl alcohol, shellac, silicon dioxide, talc, and titanium dioxide. In addition, 500 mg tablets contain iron oxide red and iron oxide yellow. Divalproex sodium extended-release tablets USP meet USP Dissolution Test 11. Image 1
How Supplied
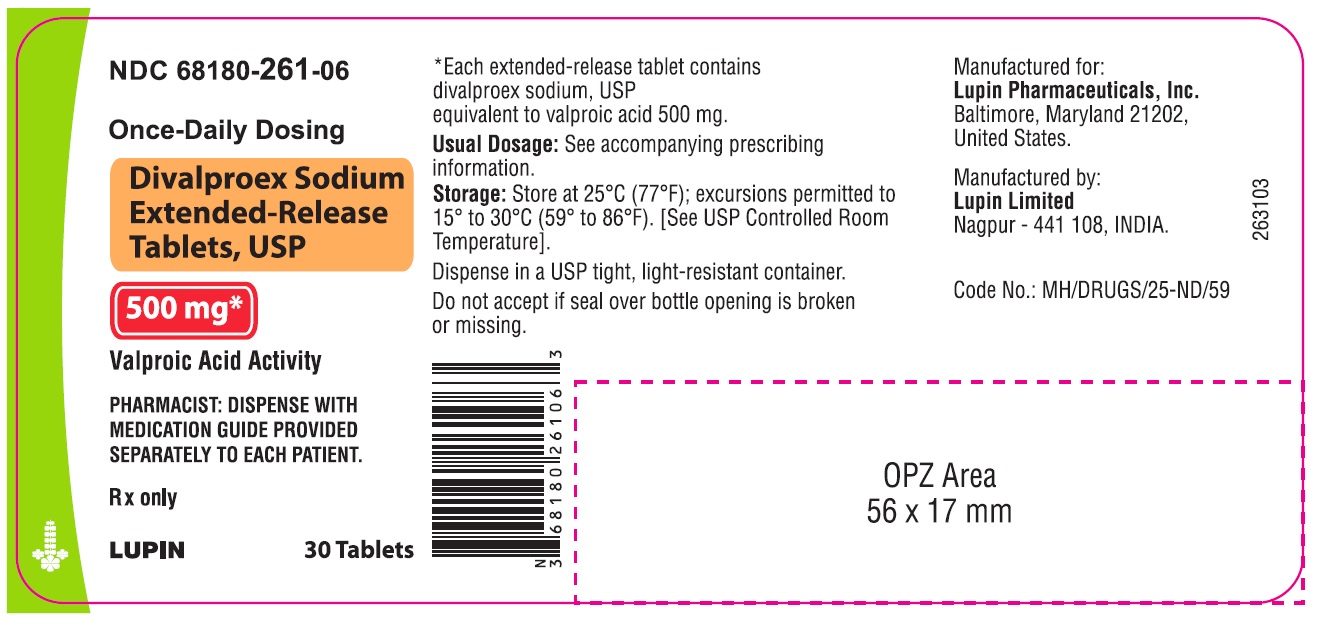
16 HOW SUPPLIED/STORAGE AND HANDLING Divalproex sodium extended-release tablets USP, 250 mg are available as white, oval shaped, film coated tablets, imprinted "L088" on one side and plain on the other side. Each divalproex sodium extended-release tablet contains divalproex sodium equivalent to 250 mg of valproic acid in the following package sizes: Bottle of 30 (NDC 68180-260-06) Bottle of 100 (NDC 68180-260-01) Bottle of 500 (NDC 68180-260-02) Divalproex sodium extended-release tablets USP, 500 mg are available as grey colored, oval shaped, film coated tablets, imprinted "L089" on one side and plain on the other side. Each divalproex sodium extended-release tablet contains divalproex sodium equivalent to 500 mg of valproic acid in the following packaging sizes: Bottle of 30 (NDC 68180-261-06) Bottle of 100 (NDC 68180-261-01) Bottle of 500 (NDC 68180-261-02) Recommended Storage Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]. Dispense in a USP tight, light-resistant container.
Indications & Usage
1 INDICATIONS AND USAGE Divalproex sodium extended-release tablets are indicated for: Acute treatment of manic or mixed episodes associated with bipolar disorder, with or without psychotic features ( 1.1 ) Monotherapy and adjunctive therapy of complex partial seizures and simple and complex absence seizures; adjunctive therapy in patients with multiple seizure types that include absence seizures ( 1.2) Prophylaxis of migraine headaches ( 1.3) 1.1 Mania Divalproex sodium extended-release tablets are valproate and are indicated for the treatment of acute manic or mixed episodes associated with bipolar disorder, with or without psychotic features. A manic episode is a distinct period of abnormally and persistently elevated, expansive, or irritable mood. Typical symptoms of mania include pressure of speech, motor hyperactivity, reduced need for sleep, flight of ideas, grandiosity, poor judgment, aggressiveness, and possible hostility. A mixed episode is characterized by the criteria for a manic episode in conjunction with those for a major depressive episode (depressed mood, loss of interest or pleasure in nearly all activities). The efficacy of divalproex sodium extended-release tablets are based in part on studies of divalproex sodium delayed-release tablets in this indication, and was confirmed in a 3-week trial with patients meeting DSM-IV TR criteria for bipolar I disorder, manic or mixed type, who were hospitalized for acute mania [see Clinical Studies ( 14.1 )] . The effectiveness of valproate for long-term use in mania, i.e., more than 3 weeks, has not been demonstrated in controlled clinical trials. Therefore, healthcare providers who elect to use divalproex sodium extended-release tablets for extended periods should continually reevaluate the long-term risk-benefits of the drug for the individual patient. 1.2 Epilepsy Divalproex sodium extended-release tablets are indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the age of 10 years with complex partial seizures that occur either in isolation or in association with other types of seizures. Divalproex sodium extended-release tablets are also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures in adults and children 10 years of age or older, and adjunctively in adults and children 10 years of age or older with multiple seizure types that include absence seizures. Simple absence is defined as very brief clouding of the sensorium or loss of consciousness accompanied by certain generalized epileptic discharges without other detectable clinical signs. Complex absence is the term used when other signs are also present. 1.3 Migraine Divalproex sodium extended-release tablets are indicated for prophylaxis of migraine headaches. There is no evidence that divalproex sodium extended-release tablets are useful in the acute treatment of migraine headaches. 1.4 Important Limitations Because of the risk to the fetus of decreased IQ, neurodevelopmental disorders, neural tube defects, and other major congenital malformations, which may occur very early in pregnancy, valproate should not be used to treat women with epilepsy or bipolar disorder who are pregnant or who plan to become pregnant unless other medications have failed to provide adequate symptom control or are otherwise unacceptable. Valproate should not be administered to a woman of childbearing potential unless other medications have failed to provide adequate symptom control or are otherwise unacceptable [see Warnings and Precautions ( 5.2 , 5.3 , 5.4 ) , Use in Specific Populations ( 8.1 ), and Patient Counseling Information (17)] . For prophylaxis of migraine headaches, divalproex sodium extended-release tablet is contraindicated in women who are pregnant and in women of childbearing potential who are not using effective contraception [see Contraindications ( 4 )] .
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Divalproex sodium extended-release tablets are intended for once-a-day oral administration. Divalproex sodium extended-release tablets should be swallowed whole and should not be crushed or chewed ( 2.1 , 2.2 ). Mania: Initial dose is 25 mg/kg/day, increasing as rapidly as possible to achieve therapeutic response or desired plasma level (2.1). The maximum recommended dosage is 60 mg/kg/day ( 2.1 , 2.2 ). Complex Partial Seizures: Start at 10 to 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day to achieve optimal clinical response; if response is not satisfactory, check valproate plasma level; see full prescribing information for conversion to monotherapy ( 2.2 ). The maximum recommended dosage is 60 mg/kg/day ( 2.1 , 2.2 ). Absence Seizures: Start at 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day until seizure control or limiting side effects (2.2). The maximum recommended dosage is 60 mg/kg/day ( 2.1 , 2.2 ). Migraine: The recommended starting dose is 500 mg/day for 1 week, thereafter increasing to 1,000 mg/day ( 2.3 ). Divalproex sodium extended-release tablet is an extended-release product intended for once-a-day oral administration. Divalproex sodium extended-release tablets should be swallowed whole and should not be crushed or chewed. 2.1 Mania Divalproex sodium extended-release tablets are administered orally. The recommended initial dose is 25 mg/kg/day given once daily. The dose should be increased as rapidly as possible to achieve the lowest therapeutic dose which produces the desired clinical effect or the desired range of plasma concentrations. In a placebo-controlled clinical trial of acute mania or mixed type, patients were dosed to a clinical response with a trough plasma concentration between 85 and 125 mcg/mL. The maximum recommended dosage is 60 mg/kg/day. There is no body of evidence available from controlled trials to guide a clinician in the longer term management of a patient who improves during divalproex sodium extended-release tablet treatment of an acute manic episode. While it is generally agreed that pharmacological treatment beyond an acute response in mania is desirable, both for maintenance of the initial response and for prevention of new manic episodes, there are no data to support the benefits of divalproex sodium extended-release tablets in such longer-term treatment (i.e., beyond 3 weeks). 2.2 Epilepsy Divalproex sodium extended-release tablets are administered orally, and must be swallowed whole. As divalproex sodium extended-release tablets dosage is titrated upward, concentrations of clonazepam, diazepam, ethosuximide, lamotrigine, tolbutamide, phenobarbital, carbamazepine, and/or phenytoin may be affected [see Drug Interactions ( 7.2 )] . Complex Partial Seizures For adults and children 10 years of age or older. Monotherapy (Initial Therapy): Divalproex sodium extended-release tablets have not been systematically studied as initial therapy. Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. The probability of thrombocytopenia increases significantly at total trough valproate plasma concentrations above 110 mcg/mL in females and 135 mcg/mL in males. The benefit of improved seizure control with higher doses should be weighed against the possibility of a greater incidence of adverse reactions. Conversion to Monotherapy: Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. Concomitant antiepilepsy drug (AED) dosage can ordinarily be reduced by approximately 25% every 2 weeks. This reduction may be started at initiation of divalproex sodium extended-release tablets therapy, or delayed by 1 to 2 weeks if there is a concern that seizures are likely to occur with a reduction. The speed and duration of withdrawal of the concomitant AED can be highly variable, and patients should be monitored closely during this period for increased seizure frequency. Adjunctive Therapy: Divalproex sodium extended-release tablets may be added to the patient's regimen at a dosage of 10 to 15 mg/kg/day. The dosage may be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. In a study of adjunctive therapy for complex partial seizures in which patients were receiving either carbamazepine or phenytoin in addition to valproate, no adjustment of carbamazepine or phenytoin dosage was needed [see Clinical Studies ( 14.2 )] . However, since valproate may interact with these or other concurrently administered AEDs as well as other drugs, periodic plasma concentration determinations of concomitant AEDs are recommended during the early course of therapy [see Drug Interactions ( 7 )] . Simple and Complex Absence Seizures The recommended initial dose is 15 mg/kg/day, increasing at one week intervals by 5 to 10 mg/kg/day until seizures are controlled or side effects preclude further increases. The maximum recommended dosage is 60 mg/kg/day. A good correlation has not been established between daily dose, serum concentrations, and therapeutic effect. However, therapeutic valproate serum concentration for most patients with absence seizures is considered to range from 50 to 100 mcg/mL. Some patients may be controlled with lower or higher serum concentrations [see Clinical Pharmacology ( 12.3 )] . As divalproex sodium extended-release tablets dosage is titrated upward, blood concentrations of phenobarbital and/or phenytoin may be affected [see Drug Interactions ( 7.2 )] . Antiepilepsy drugs should not be abruptly discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. 2.3 Migraine Divalproex sodium extended-release tablets are indicated for prophylaxis of migraine headaches in adults. The recommended starting dose is 500 mg once daily for 1 week, thereafter increasing to 1,000 mg once daily. Although doses other than 1,000 mg once daily of divalproex sodium extended-release tablets have not been evaluated in patients with migraine, the effective dose range of divalproex sodium delayed-release tablets in these patients is 500 to 1,000 mg/day. As with other valproate products, doses of divalproex sodium extended-release tablets should be individualized and dose adjustment may be necessary. If a patient requires smaller dose adjustments than that available with divalproex sodium extended-release tablets, divalproex sodium delayed-release tablets should be used instead. 2.4 Conversion from Divalproex Sodium Delayed-Release Tablets to Divalproex Sodium Extended-Release Tablets In adult patients and pediatric patients 10 years of age or older with epilepsy previously receiving divalproex sodium delayed-release tablets, divalproex sodium extended-release tablets should be administered once-daily using a dose 8 to 20% higher than the total daily dose of divalproex sodium delayed-release tablets (Table 1). For patients whose divalproex sodium delayed-release tablets total daily dose cannot be directly converted to divalproex sodium extended-release tablets, consideration may be given at the clinician's discretion to increase the patient's divalproex sodium delayed-release tablets total daily dose to the next higher dosage before converting to the appropriate total daily dose of divalproex sodium extended-release tablets. Table 1. Dose Conversion Divalproex Sodium Delayed-Release Tablets Divalproex Sodium Extended-Release Tablets Total Daily Dose (mg) (mg) 500 These total daily doses of divalproex sodium delayed-release tablets cannot be directly converted to an 8 to 20% higher total daily dose of divalproex sodium extended-release tablets because the required dosing strengths of divalproex sodium extended-release tablets are not available. Consideration may be given at the clinician's discretion to increase the patient's divalproex sodium delayed-release tablets total daily dose to the next higher dosage before converting to the appropriate total daily dose of divalproex sodium extended-release tablets. to 625 750 750 to 875 1,000 1,000 to 1,125 1,250 1,250 to 1,375 1,500 1,500 to 1,625 1,750 1,750 2,000 1,875 to 2,000 2,250 2,125 to 2,250 2,500 2,375 2,750 2,500 to 2,750 3,000 2,875 3,250 3,000 to 3,125 3,500 There is insufficient data to allow a conversion factor recommendation for patients with divalproex sodium delayed-release tablets doses above 3,125 mg/day. Plasma valproate C min concentrations for divalproex sodium extended-release tablets on average are equivalent to divalproex sodium delayed-release tablets, but may vary across patients after conversion. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL) [see Clinical Pharmacology ( 12.2 )] . 2.5 General Dosing Advice Dosing in Elderly Patients Due to a decrease in unbound clearance of valproate and possibly a greater sensitivity to somnolence in the elderly, the starting dose should be reduced in these patients. Starting doses in the elderly lower than 250 mg can only be achieved by the use of divalproex sodium delayed-release tablets. Dosage should be increased more slowly and with regular monitoring for fluid and nutritional intake, dehydration, somnolence, and other adverse reactions. Dose reductions or discontinuation of valproate should be considered in patients with decreased food or fluid intake and in patients with excessive somnolence. The ultimate therapeutic dose should be achieved on the basis of both tolerability and clinical response [see Warnings and Precautions ( 5.14 ), Use in Specific Populations ( 8.5 ), and Clinical Pharmacology ( 12.3 )] . Dose-Related Adverse Reactions The frequency of adverse effects (particularly elevated liver enzymes and thrombocytopenia) may be dose-related. The probability of thrombocytopenia appears to increase significantly at total valproate concentrations of ≥ 110 mcg/mL (females) or ≥ 135 mcg/mL (males) [see Warnings and Precautions ( 5.8 )] . The benefit of improved therapeutic effect with higher doses should be weighed against the possibility of a greater incidence of adverse reactions. G. I. Irritation Patients who experience G.I. irritation may benefit from administration of the drug with food or by slowly building up the dose from an initial low level. Compliance Patients should be informed to take divalproex sodium extended-release tablets every day as prescribed. If a dose is missed it should be taken as soon as possible, unless it is almost time for the next dose. If a dose is skipped, the patient should not double the next dose. 2.6 Dosing in Patients Taking Rufinamide Patients stabilized on rufinamide before being prescribed valproate should begin valproate therapy at a low dose, and titrate to a clinically effective dose [see Drug Interactions ( 7.2 ) ] .
