Drug Catalog - Product Detail
DONEPEZIL HCL ODT TB 10MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68382-0347-06 | ZYDUS PHARMACEUTICALS (USA) | 30 | 10MG | TABLET |
PACKAGE FILES



Generic Name
DONEPEZIL HYDROCHLORIDE
Substance Name
DONEPEZIL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA090175
Description
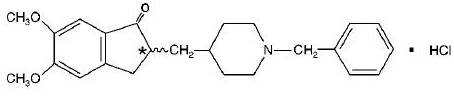
11 DESCRIPTION Donepezil hydrochloride is a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2, 3-dihydro-5, 6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1 H -inden-1-one hydrochloride. Donepezil hydrochloride is commonly referred to in the pharmacological literature as E2020. It has a molecular formula of C 24 H 29 NO 3 HCl and a molecular weight of 415.96. Donepezil hydrochloride, USP is a white to off-white crystalline powder. It is freely soluble in chloroform, soluble in water and in glacial acetic acid, slightly soluble in ethanol and in acetonitrile and practically insoluble in ethyl acetate and in n-hexane. Each donepezil hydrochloride orally disintegrating tablet, USP intended for oral administration contains 5 mg or 10 mg of donepezil hydrochloride. In addition, each tablet contains the following inactive ingredients: ammonium glycyrrhizate, colloidal silicon dioxide, crospovidone, flavor peppermint, flavor strawberry, magnesium stearate, mannitol and sucralose. Image
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Donepezil Hydrochloride Orally disintegrating Tablets USP, 5 mg are white to off-white, round-shaped, biconvex, uncoated tablets engraved with 'ZF 14'on one side and plain on other side and are supplied as follows: NDC 68382-346-06 in bottle of 30 tablets NDC 68382-346-16 in bottle of 90 tablets NDC 68382-346-01 in bottle of 100 tablets NDC 68382-346-05 in bottle of 500 tablets NDC 68382-346-10 in bottle of 1000 tablets NDC 68382-346-77 in unit-dose blister cartons of 100 (10 x 10) unit dose tablets Donepezil Hydrochloride Orally Disintegrating Tablets USP, 10 mg are white to off-white, round-shaped, biconvex, uncoated tablets engraved with 'ZF 15'on one side and plain on other side and are supplied as follows: NDC 68382-347-06 in bottle of 30 tablets NDC 68382-347-16 in bottle of 90 tablets NDC 68382-347-01 in bottle of 100 tablets NDC 68382-347-05 in bottle of 500 tablets NDC 68382-347-10 in bottle of 1000 tablets NDC 68382-347-77 in unit-dose blister cartons of 100 (10 x 10) unit dose tablets Storage Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight container.
Indications & Usage
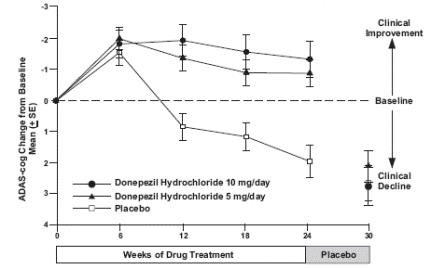
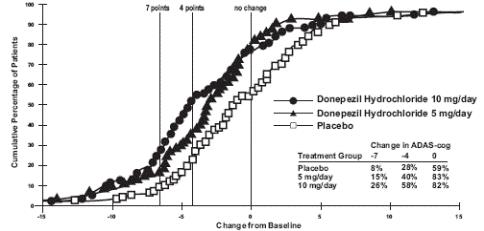
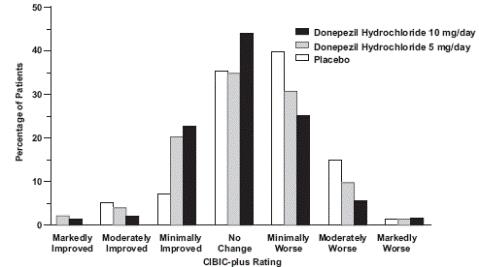
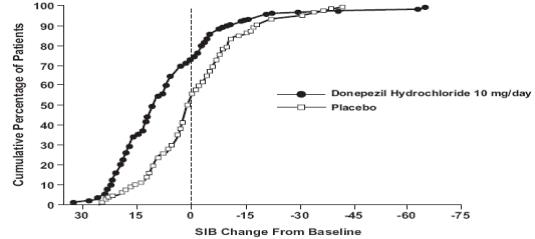
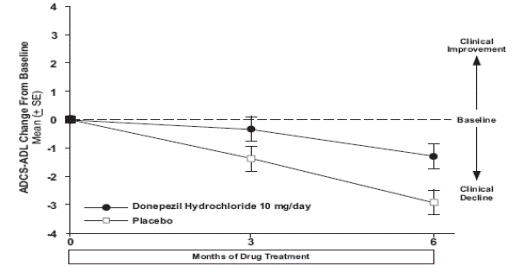
1 INDICATIONS AND USAGE Donepezil hydrochloride orally disintegrating tablets are indicated for the treatment of dementia of the Alzheimer's type. Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer's disease. Donepezil hydrochloride orally disintegrating tablets are an acetylcholinesterase inhibitor indicated for the treatment of dementia of the Alzheimer's type. Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer's Disease ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Mild to Moderate Alzheimer's Disease: 5 mg to 10 mg once daily ( 2.1 ) Moderate to Severe Alzheimer's Disease: 10 mg once daily ( 2.2 ) 2.1 Dosing in Mild to Moderate Alzheimer’s Disease The recommended starting dosage of donepezil hydrochloride is 5 mg administered once per day in the evening, just prior to retiring. The maximum recommended dosage of donepezil hydrochloride in patients with mild to moderate Alzheimer's disease is 10 mg per day. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks. 2.2 Dosing in Moderate to Severe Alzheimer’s Disease The recommended starting dosage of donepezil hydrochloride is 5 mg administered once per day in the evening, just prior to retiring. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks. 2.3 Administration Information Donepezil hydrochloride orally disintegrating tablets should be taken in the evening, just prior to retiring. Donepezil hydrochloride orally disintegrating tablets can be taken with or without food. Allow donepezil hydrochloride orally disintegrating tablets to dissolve on the tongue and follow with water.
