Drug Catalog - Product Detail
DONEPEZIL HCL TB 23MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 24979-0004-06 | TWI PHARMACEUTICALS | 30 | 23MG | TABLET |
PACKAGE FILES

















Generic Name
DONEPEZIL HYDROCHLORIDE
Substance Name
DONEPEZIL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA203104
Description
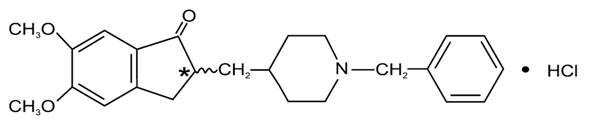
11 DESCRIPTION Donepezil hydrochloride tablets USP are a reversible inhibitor of the enzyme acetylcholinesterase, known chemically as (±)-2, 3-dihydro-5, 6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one hydrochloride. Donepezil hydrochloride is commonly referred to in the pharmacological literature as E2020. It has an empirical formula of C 24 H 29 NO 3 HCl and a molecular weight of 415.96. Donepezil hydrochloride is a white crystalline powder and is freely soluble in chloroform, soluble in water and in glacial acetic acid, slightly soluble in ethanol and in acetonitrile, and practically insoluble in ethyl acetate and in n-hexane. Donepezil hydrochloride tablets USP are available for oral administration in film-coated tablets containing 23 mg of donepezil hydrochloride. Inactive ingredients in 23 mg tablets include hypromellose, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, methacrylic acid copolymer, polyvinyl alcohol, polyethylene glycol, titanium dioxide, talc, sodium hydroxide, triethyl citrate, iron oxide black, and propylene glycol. USP Dissolution Test pending. USP Organic Impurities Test pending. 01
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 Donepezil Hydrochloride Tablets USP Supplied as film-coated, round tablets containing 23 mg of donepezil hydrochloride. The 23 mg film-coated tablets are white, printed with “T004” in black ink on one side and plain on the other side. Bottles of 30 (NDC# 24979-004-06) Bottles of 90 (NDC# 24979-004-07) Storage Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Donepezil hydrochloride tablets are indicated for the treatment of dementia of the Alzheimer’s type. Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer’s disease. Donepezil hydrochloride tablets are acetylcholinesterase inhibitor indicated for the treatment of dementia of the Alzheimer’s type. Efficacy has been demonstrated in patients with mild, moderate, and severe Alzheimer’s Disease ( 1 )
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Mild to Moderate Alzheimer’s Disease: 5 mg to 10 mg once daily ( 2.1 ) Moderate to Severe Alzheimer’s Disease: 10 mg to 23 mg once daily ( 2.2 ) 2.1 Dosing in Mild to Moderate Alzheimer’s Disease The recommended starting dosage of donepezil hydrochloride tablets is 5 mg administered once per day in the evening, just prior to retiring. The maximum recommended dosage of donepezil hydrochloride tablets in patients with mild to moderate Alzheimer’s disease is 10 mg per day. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks. 2.2 Dosing in Moderate to Severe Alzheimer’s Disease The recommended starting dosage of donepezil hydrochloride tablets is 5 mg administered once per day in the evening, just prior to retiring. The maximum recommended dosage of donepezil hydrochloride tablets in patients with moderate to severe Alzheimer’s disease is 23 mg per day. A dose of 10 mg should not be administered until patients have been on a daily dose of 5 mg for 4 to 6 weeks. A dose of 23 mg per day should not be administered until patients have been on a daily dose of 10 mg for at least 3 months. 2.3 Administration Information Donepezil hydrochloride tablets should be taken in the evening, just prior to retiring. Donepezil hydrochloride tablets can be taken with or without food. The donepezil hydrochloride 23 mg tablet should not be split, crushed, or chewed.
