Drug Catalog - Product Detail
DOXEPIN HCL 50MG CP 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 59651-0175-01 | AUROBINDO PHARMA | 100 | 50MG | CAPSULE |
PACKAGE FILES

Generic Name
Substance Name
Product Type
Route
Application Number
Description
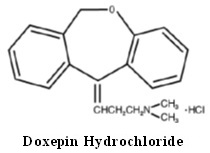
DESCRIPTION Doxepin hydrochloride is one of a class of psychotherapeutic agents known as dibenzoxepin tricyclic compounds. The molecular formula of the compound is C 19 H 21 NO•HCl having a molecular weight of 315.84. It is a white or almost white crystalline powder freely soluble in water, in alcohol and in methylene chloride. Its structural formula is: Chemically, doxepin hydrochloride is a dibenzoxepin derivative and is the first of a family of tricyclic psychotherapeutic agents. Specifically, it is an isomeric mixture of: 1-Propanamine, 3-dibenz[b,e]oxepin-11(6H)ylidene-N,N-dimethyl-, hydrochloride. Each 10 mg, 25 mg, 50 mg, 75 mg and 100 mg of doxepin hydrochloride capsule USP contains doxepin hydrochloride USP is equivalent to 10 mg, 25 mg, 50 mg, 75 mg and 100 mg of doxepin, respectively for oral administration. Each capsule contains the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, pregelatinized starch (maize) and sodium lauryl sulfate. The empty hard gelatin capsule shells contain D&C Yellow No. 10, gelatin, sodium lauryl sulfate and titanium dioxide. In addition, 10 mg, 25 mg and 50 mg contain FD&C Yellow No. 6 and 75 mg and 100 mg contain FD&C Green No. 3. The imprinting ink contains black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution. FDA approved dissolution test specifications differ from USP. Chemical Structure
How Supplied
HOW SUPPLIED Doxepin Hydrochloride Capsules, USP are available containing Doxepin Hydrochloride, USP equivalent to 10 mg, 25 mg, 50 mg, 75 mg or 100 mg of Doxepin. Doxepin Hydrochloride Capsules USP, 10 mg are buff colored size “4” hard gelatin capsule filled with white to off-white granules and imprinted with DOX on cap and 10 on body. Bottles of 100 NDC 59651-173-01 Doxepin Hydrochloride Capsules USP, 25 mg are ivory and white colored size “3” hard gelatin capsule filled with white to off-white granules and imprinted with DOX on cap and 25 on body. Bottles of 100 NDC 59651-174-01 Doxepin Hydrochloride Capsules USP, 50 mg are ivory colored size “3” hard gelatin capsule filled with white to off-white granules and imprinted with DOX on cap and 50 on body. Bottles of 100 NDC 59651-175-01 Doxepin Hydrochloride Capsules USP, 75 mg are brite lite green colored size “2” hard gelatin capsule filled with white to off-white granules and imprinted with DOX on cap and 75 on body. Bottles of 100 NDC 59651-176-01 Doxepin Hydrochloride Capsules USP, 100 mg are brite lite green and white colored size “1” hard gelatin capsule filled with white to off-white granules and imprinted with DOX on cap and 100 on body. Bottles of 100 NDC 59651-177-01 Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Protect from light. Dispense with Medication Guide available at: www.aurobindousa.com/product-medication-guides Distributed by: Aurobindo Pharma USA, Inc. 279 Princeton-Hightstown Road East Windsor, NJ 08520 Manufactured by: Aurobindo Pharma Limited Hyderabad-500 038, India Issued: December 2018
Indications & Usage
INDICATIONS AND USAGE Doxepin hydrochloride capsules are recommended for the treatment of: 1. Psychoneurotic patients with depression and/or anxiety. 2. Depression and/or anxiety associated with alcoholism (not to be taken concomitantly with alcohol). 3. Depression and/or anxiety associated with organic disease (the possibility of drug interaction should be considered if the patient is receiving other drugs concomitantly). 4. Psychotic depressive disorders with associated anxiety including involutional depression and manic-depressive disorders. The target symptoms of psychoneurosis that respond particularly well to doxepin hydrochloride capsules include anxiety, tension, depression, somatic symptoms and concerns, sleep disturbances, guilt, lack of energy, fear, apprehension and worry. Clinical experience has shown that doxepin hydrochloride capsules are safe and well tolerated even in the elderly patient. Owing to lack of clinical experience in the pediatric population, doxepin hydrochloride capsules are not recommended for use in children under 12 years of age.
Dosage and Administration
DOSAGE AND ADMINISTRATION For most patients with illness of mild to moderate severity, a starting daily dose of 75 mg is recommended. Dosage may subsequently be increased or decreased at appropriate intervals and according to individual response. The usual optimum dose range is 75 mg/day to 150 mg/day. In more severely ill patients higher doses may be required with subsequent gradual increase to 300 mg/day if necessary. Additional therapeutic effect is rarely to be obtained by exceeding a dose of 300 mg/day. In patients with very mild symptomatology or emotional symptoms accompanying organic disease, lower doses may suffice. Some of these patients have been controlled on doses as low as 25 to 50 mg/day. The total daily dosage of doxepin hydrochloride may be given on a divided or once-a-day dosage schedule. If the once-a-day schedule is employed, the maximum recommended dose is 150 mg/day. This dose may be given at bedtime. The 150 mg capsule strength is intended for maintenance therapy only and is not recommended for initiation of treatment. Anti-anxiety effect is apparent before the antidepressant effect. Optimal antidepressant effect may not be evident for two to three weeks.
