Drug Catalog - Product Detail
ENTECAVIR TABS 1MG 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 31722-0834-30 | CAMBER PHARMACEUTICALS | 30 | 1MG | TABLET |
PACKAGE FILES




Generic Name
ENTECAVIR
Substance Name
ENTECAVIR ANHYDROUS
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA205740
Description
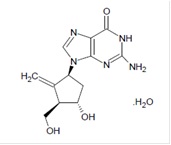
11 DESCRIPTION Entecavir tablet USP, is a guanosine nucleoside analogue with selective activity against HBV. The chemical name for entecavir is 2-Amino-1,9-dihydro-9-[(1S, 3R, 4S)-4-hydroxy-3-(Hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one, Monohydrate. Its molecular formula is C 12 H 15 N 5 O 3 •H 2 O, which corresponds to a molecular weight of 295.29. Entecavir has the following structural formula: Entecavir USP is an off-white to white color powder. It is soluble in dimethyl sulfoxide. Entecavir film-coated tablets are available for oral administration in strengths of 0.5 mg and 1 mg of entecavir. Entecavir 0.5 mg and 1 mg film-coated tablets contain the following inactive ingredients: calcium carbonate, carboxymethyl cellulose sodium, citric acid monohydrate, pregelatinized starch, sodium stearyl fumarate and soy polysaccharides. The tablet coating contains hypromellose, iron oxide red (1 mg tablet only), polyethylene glycol, polysorbate 80 (0.5 mg tablet only) and titanium dioxide. Fig1.jpg
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Entecavir tablets USP, 0.5 mg are white to off white, triangle shaped, biconvex, film-coated tablets, debossed with ʽJʼ on one side and ʽ110ʼ on the other side and supplied as: Bottles of 30 tablets NDC 31722-833-30 Bottles of 90 tablets NDC 31722-833-90 Blister card of 10 unit dose tablets NDC 31722-833-31 Blister pack of 100 (10 x 10) unit dose tablets NDC 31722-833-32 Entecavir tablets USP, 1 mg are pink, triangle shaped, biconvex, film-coated tablets, debossed with ʽJʼ on one side and ʽ111ʼ on the other side and supplied as: Bottles of 30 tablets NDC 31722-834-30 Bottles of 90 tablets NDC 31722-834-90 Blister card of 8 unit dose tablets NDC 31722-834-31 Blister pack of 80 (10 x 8) unit dose tablets NDC 31722-834-32 Storage Entecavir tablets should be stored in a tightly closed container at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
Indications & Usage
1 INDICATIONS AND USAGE Entecavir tablets are indicated for the treatment of chronic hepatitis B virus infection in adults and pediatric patients 2 years of age and older with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. Entecavir is a hepatitis B virus nucleoside analogue reverse transcriptase inhibitor indicated for the treatment of chronic hepatitis B virus infection in adults and children at least 2 years of age with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease. (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION • Nucleoside-inhibitor-treatment-naïve with compensated liver disease (greater than or equal to 16 years old): 0.5 mg once daily. (2.2) • Nucleoside-inhibitor-treatment-naïve and lamivudine-experienced pediatric patients at least 2 years of age and weighing at least 10 kg: dosing is based on weight. (2.3) • Lamivudine-refractory or known lamivudine or telbivudine resistance substitutions (greater than or equal to 16 years old): 1 mg once daily. (2.2) • Decompensated liver disease (adults): 1 mg once daily. (2.2) • Renal impairment: Dosage adjustment is recommended if creatinine clearance is less than 50 mL/min. (2.4) • Entecavir tablets should be administered on an empty stomach. (2.1) 2.1 Timing of Administration Entecavir tablets should be administered on an empty stomach (at least 2 hours after a meal and 2 hours before the next meal). 2.2 Recommended Dosage in Adults Compensated Liver Disease The recommended dose of entecavir tablets for chronic hepatitis B virus infection in nucleoside-inhibitor-treatment-naïve adults and adolescents 16 years of age and older is 0.5 mg once daily. The recommended dose of entecavir tablets in adults and adolescents (at least 16 years of age) with a history of hepatitis B viremia while receiving lamivudine or known lamivudine or telbivudine resistance substitutions rtM204I/V with or without rtL180M, rtL80I/V, or rtV173L is 1 mg once daily. Decompensated Liver Disease The recommended dose of entecavir tablets for chronic hepatitis B virus infection in adults with decompensated liver disease is 1 mg once daily. 2.3 Recommended Dosage in Pediatric Patients Table 1 describes the recommended dose of entecavir tablets for pediatric patients 2 years of age or older and weighing at least 10 kg. The oral solution should be used for patients with body weight up to 30 kg. Table 1: Dosing Schedule for Pediatric Patients Recommended Once-Daily Dose of Oral Solution (mL) Body Weight (kg) Treatment-Na ï ve Patients a Lamivudine-Experienced Patients b 10 to 11 3 6 greater than 11 to 14 4 8 greater than 14 to 17 5 10 greater than 17 to 20 6 12 greater than 20 to 23 7 14 greater than 23 to 26 8 16 greater than 26 to 30 9 18 greater than 30 10 20 a Children with body weight greater than 30 kg should receive 10 mL (0.5 mg) of oral solution or one 0.5 mg tablet once daily. b Children with body weight greater than 30 kg should receive 20 mL (1 mg) of oral solution or one 1 mg tablet once daily. 2.4 Renal Impairment In adult subjects with renal impairment, the apparent oral clearance of entecavir decreased as creatinine clearance decreased [see Clinical Pharmacology ( 12.3 )]. Dosage adjustment is recommended for patients with creatinine clearance less than 50 mL/min, including patients on hemodialysis or continuous ambulatory peritoneal dialysis (CAPD), as shown in Table 2. The once-daily dosing regimens are preferred. Table 2: Recommended Dosage of Entecavir Tablets in Adult Patients with Renal Impairment Creatinine Clearance (mL/min) Usual Dose (0.5 mg) Lamivudine-Refractory or Decompensated Liver Disease (1 mg) 50 or greater 0.5 mg once daily 1 mg once daily 30 to less than 50 0.5 mg every 48 hours 0.5 mg once daily O r 1 mg every 48 hours 10 to less than 30 0.5 mg every 72 hours 1 mg every 72 hours Less than 10 Hemodialysis b or CAPD 0.5 mg every 7 days 1 mg every 7 days b If administered on a hemodialysis day, administer entecavir tablets after the hemodialysis session. Although there are insufficient data to recommend a specific dose adjustment of entecavir tablets in pediatric patients with renal impairment, a reduction in the dose or an increase in the dosing interval similar to adjustments for adults should be considered. 2.5 Hepatic Impairment No dosage adjustment is necessary for patients with hepatic impairment. 2.6 Duration of Therapy The optimal duration of treatment with entecavir tablets for patients with chronic hepatitis B virus infection and the relationship between treatment and long-term outcomes such as cirrhosis and hepatocellular carcinoma are unknown.
