Drug Catalog - Product Detail
ESMOLOL HCL FOR INJECTION INJECT. 100MG/10ML 1X25
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 55150-0194-10 | AUROMEDICS PHARMA | 10 | 100MG/10ML | SOLUTION |
PACKAGE FILES


Generic Name
ESMOLOL HYDROCHLORIDE
Substance Name
ESMOLOL HYDROCHLORIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAVENOUS
Application Number
ANDA205520
Description
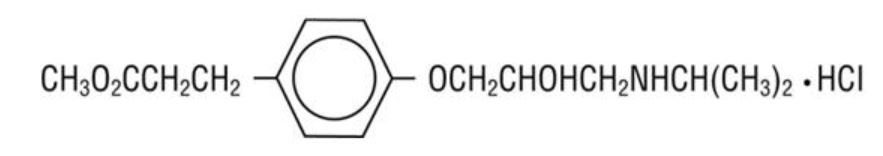
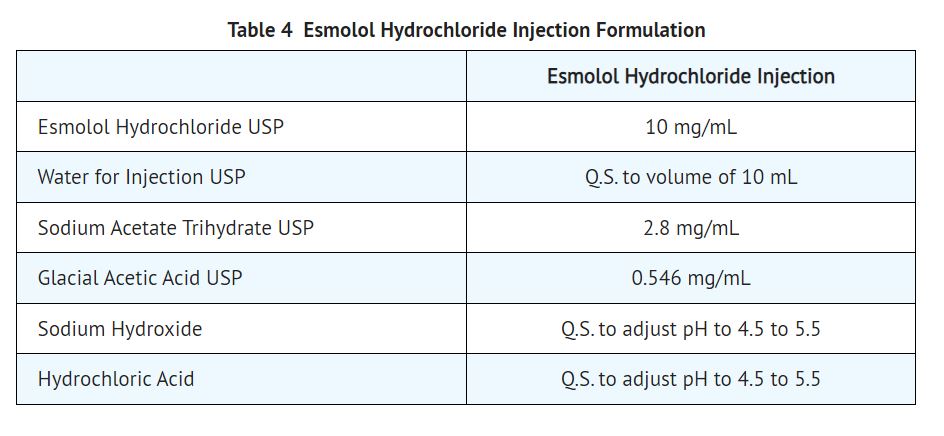
11 DESCRIPTION Esmolol hydrochloride injection is a beta adrenergic receptor blocker with a very short duration of action (elimination half-life is approximately 9 minutes). Esmolol hydrochloride is: (±)-Methyl p-[2-hydroxy-3-(isopropylamino) propoxy] hydrocinnamate hydrochloride and has the following structure: Esmolol hydrochloride, USP has the molecular formula C 16 H 26 NO 4 Cl and a molecular weight of 331.8. It has one asymmetric center and exists as an enantiomeric pair. Esmolol hydrochloride, USP is a white to off-white crystalline powder. It is a relatively hydrophilic compound which is very soluble in water and freely soluble in alcohol. Its partition coefficient (octanol/water) at pH 7.0 is 0.42 compared to 17 for propranolol. 11.1 Esmolol Hydrochloride Injection Dosage Forms All Esmolol hydrochloride injection presentations are a clear, colorless to light yellow, sterile, nonpyrogenic solution of esmolol hydrochloride. The formulation for esmolol hydrochloride in sodium chloride injection and esmolol hydrochloride injection is described in the table below: The calculated osmolarity of esmolol hydrochloride in sodium chloride injection and esmolol hydrochloride in sodium chloride injection double strength is 312 mOsmol/L. The 250 mL and 100 mL bags are non-latex, non-PVC flexible containers with dual polypropylene ports. The flexible containers are manufactured from a specially designed multilayer polypropylene. Solutions in contact with the plastic container leach out certain chemical compounds from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Formula1.jpg Image5.jpg
How Supplied
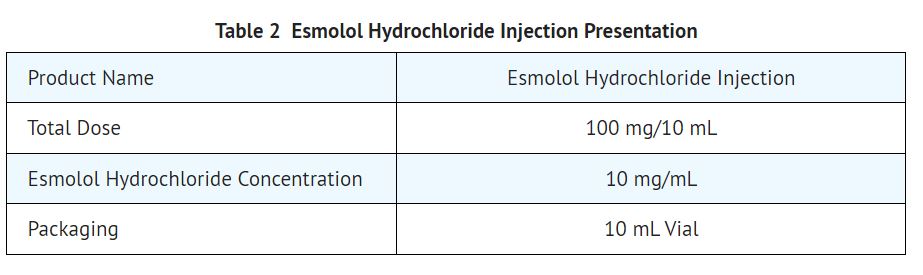
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Esmolol Hydrochloride Injection Esmolol hydrochloride injection is a clear, colorless to light yellow, sterile, nonpyrogenic solution of esmolol hydrochloride and is supplied as follows: 100 mg per 10 mL (10 mg/mL) 10 mL Single-Dose Vials in a Carton of 25 NDC 55150-194-10 Esmolol Hydrochloride in Sodium Chloride Injection Esmolol hydrochloride in sodium chloride injection is a clear, colorless to light yellow, sterile, non pyrogenic, iso-osmotic solution of esmolol hydrochloride and is supplied as follows: 2,500 mg per 250 mL (10 mg/mL) 250 mL Single-Dose Flexible Container packaged in a Carton of 10 NDC 55150-420-10 2,000 mg per 100 mL (20 mg/mL) 100 mL Single-Dose Flexible Container packaged in a Carton of 10 NDC 55150-421-10 Product repackaged by: Henry Schein, Inc., Bastian, VA 24314 From Original Manufacturer/Distributor's NDC and Unit of Sale To Henry Schein Repackaged Product NDC and Unit of Sale Total Strength/Total Volume (Concentration) per unit NDC 55150-194-10 10 mL Single-Dose Vials in a carton of 25 NDC 0404-9858-10 1 10 mL Single-Dose Vial in a bag (Vial bears NDC 55150-194-10) 100 mg per 10 mL (10 mg/mL) 16.2 Storage Esmolol Hydrochloride Injection Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from freezing. Avoid excessive heat. The vial stopper is not made with natural rubber latex. Esmolol Hydrochloride in Sodium Chloride Injection Store at 25°C (77°F); excursions permitted to 15˚ to 30˚C (59˚ to 86˚F) [see USP Controlled Room Temperature]. Protect from freezing. Avoid excessive heat. Each bag contains no preservative. Once drug has been withdrawn from ready-to-use bag, the bag should be used within 24 hours, with any unused portion discarded. Visually inspect the container. If the administration port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Do not use plastic containers in series connections. Such use could result in an embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed. Do not remove unit from overwrap until ready to use. Do not use if overwrap has been previously opened or damaged. The overwrap is a moisture barrier. The inner bag maintains sterility of the solution. Tear overwrap at notch and remove premixed bag. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing the inner bag firmly. If leaks are found, discard solution, as sterility may be impaired. Do not use unless the solution is clear (colorless to light yellow) and the seal is intact. Preparation for intravenous administration: Use aseptic technique. Suspend premixed bag from eyelet support. Remove plastic protector from delivery port at bottom of bag. Attach administration set. Refer to complete directions accompanying set.
Indications & Usage
1 INDICATIONS AND USAGE 1.1 Supraventricular Tachycardia or Noncompensatory Sinus Tachycardia Esmolol hydrochloride injection is indicated for the rapid control of ventricular rate in patients with atrial fibrillation or atrial flutter in perioperative, postoperative, or other emergent circumstances where short term control of ventricular rate with a short-acting agent is desirable. Esmolol hydrochloride injection is also indicated in noncompensatory sinus tachycardia where, in the physician’s judgment, the rapid heart rate requires specific intervention. Esmolol hydrochloride injection is intended for short-term use. 1.2 Intraoperative and Postoperative Tachycardia and/or Hypertension Esmolol hydrochloride injection is indicated for the short-term treatment of tachycardia and hypertension that occur during induction and tracheal intubation, during surgery, on emergence from anesthesia and in the postoperative period, when in the physician’s judgment such specific intervention is considered indicated. Use of esmolol hydrochloride injection to prevent such events is not recommended. Esmolol hydrochloride injection is a beta adrenergic blocker indicated for the short-term treatment of: Control of ventricular rate in supraventricular tachycardia including atrial fibrillation and atrial flutter and control of heart rate in noncompensatory sinus tachycardia (1.1) Control of perioperative tachycardia and hypertension (1.2)
Dosage and Administration
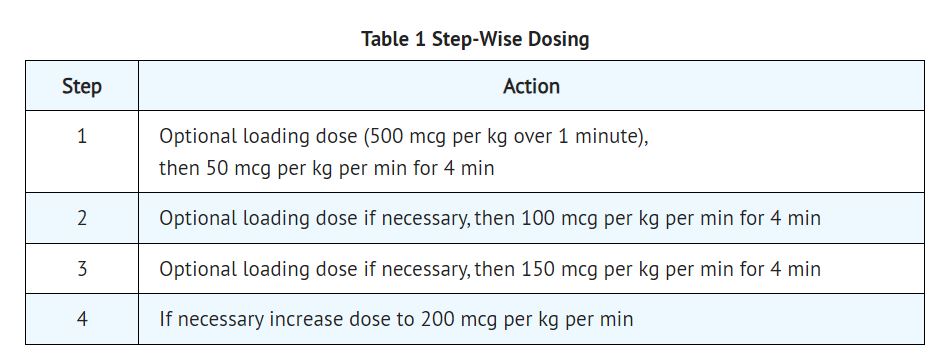
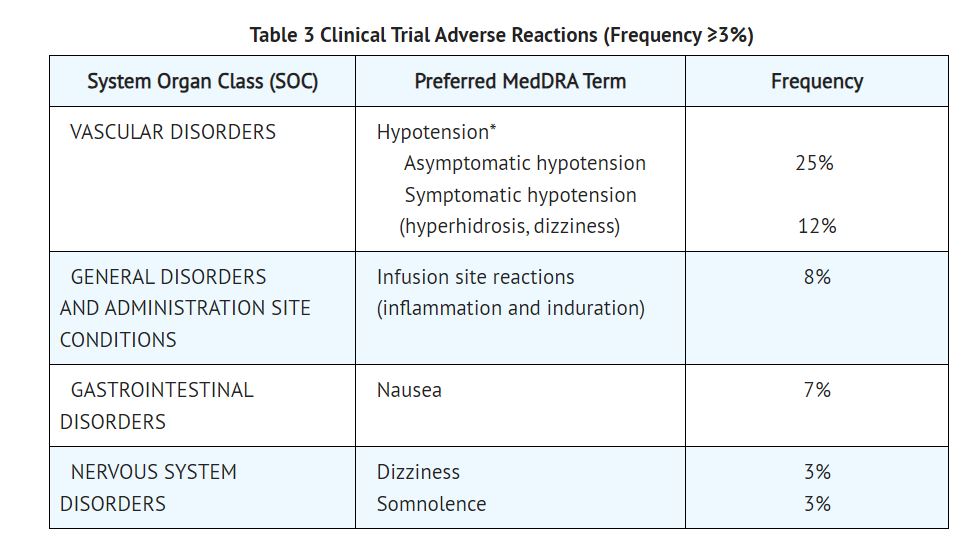
2 DOSAGE AND ADMINISTRATION 2.1 Dosing for the Treatment of Supraventricular Tachycardia or Noncompensatory Sinus Tachycardia Esmolol hydrochloride injection is administered by continuous intravenous infusion with or without a loading dose. Additional loading doses and/or titration of the maintenance infusion (step-wise dosing) may be necessary based on desired ventricular response. In the absence of loading doses, continuous infusion of a single concentration of esmolol reaches pharmacokinetic and pharmacodynamic steady-state in about 30 minutes. The effective maintenance dose for continuous and step-wise dosing is 50 to 200 mcg per kg per minute, although doses as low as 25 mcg per kg per minute have been adequate. Dosages greater than 200 mcg per kg per minute provide little added heart rate lowering effect, and the rate of adverse reactions increases. Maintenance infusions may be continued for up to 48 hours. 2.2 Intraoperative and Postoperative Tachycardia and Hypertension In this setting it is not always advisable to slowly titrate to a therapeutic effect. Therefore two dosing options are presented: immediate control and gradual control. Immediate Control Administer 1 mg per kg as a bolus dose over 30 seconds followed by an infusion of 150 mcg per kg per min if necessary. Adjust the infusion rate as required to maintain desired heart rate and blood pressure. Refer to Maximum Recommended Doses below. Gradual Control Administer 500 mcg per kg as a bolus dose over 1 minute followed by a maintenance infusion of 50 mcg per kg per min for 4 minutes. Depending on the response obtained, continue dosing as outlined for supraventricular tachycardia. Refer to Maximum Recommended Doses below. Maximum Recommended Doses For the treatment of tachycardia, maintenance infusion dosages greater than 200 mcg per kg per min are not recommended; dosages greater than 200 mcg per kg per min provide little additional heart rate-lowering effect, and the rate of adverse reactions increases. For the treatment of hypertension, higher maintenance infusion dosages (250 to 300 mcg per kg per min) may be required. The safety of doses above 300 mcg per kg per minute has not been studied. 2.3 Transition from Esmolol Hydrochloride Injection Therapy to Alternative Drugs After patients achieve adequate control of the heart rate and a stable clinical status, transition to alternative antiarrhythmic drugs may be accomplished. When transitioning from esmolol hydrochloride injection to alternative drugs, the physician should carefully consider the labeling instructions of the alternative drug selected and reduce the dosage of esmolol hydrochloride injection as follows: 1. Thirty minutes following the first dose of the alternative drug, reduce the esmolol hydrochloride infusion rate by one-half (50%). 2. After administration of the second dose of the alternative drug, monitor the patient's response and if satisfactory control is maintained for the first hour, discontinue the esmolol hydrochloride infusion. 2.4 Directions for Use Esmolol hydrochloride injection is available in a pre-mixed bag and single-dose vial. Esmolol hydrochloride injection is not compatible with Sodium Bicarbonate (5%) solution (limited stability) or furosemide (precipitation). Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Premixed Bag The medication port is to be used solely for withdrawing an initial bolus from the bag. Use aseptic technique when withdrawing the bolus dose. Do not add any additional medications to the bag. Single-Dose Vial The Single-Dose Vial may be used to administer a loading dosage by hand-held syringe while the maintenance infusion is being prepared [see How Supplied/Storage and Handling (16.2)]. Compatibility with Commonly Used Intravenous Fluids Esmolol hydrochloride injection was tested for compatibility with ten commonly used intravenous fluids at a final concentration of 10 mg esmolol hydrochloride per mL. Esmolol hydrochloride injection was found to be compatible with the following solutions and was stable for at least 24 hours at controlled room temperature or under refrigeration: Dextrose (5%) Injection, USP Dextrose (5%) in Lactated Ringer’s Injection Dextrose (5%) in Ringer’s Injection Dextrose (5%) and Sodium Chloride (0.45%) Injection, USP Dextrose (5%) and Sodium Chloride (0.9%) Injection, USP Lactated Ringer’s Injection, USP Potassium Chloride (40 mEq/liter) in Dextrose (5%) Injection, USP Sodium Chloride (0.45%) Injection, USP Sodium Chloride (0.9%) Injection, USP Administer intravenously (2.1, 2.2) Titrate using ventricular rate or blood pressure at ≥4-minute intervals. (2.1, 2.2) Supraventricular tachycardia (SVT) or noncompensatory sinus tachycardia (2.1) Optional loading dose: 500 mcg per kg infused over one minute Then 50 mcg per kg per minute for the next 4 minutes Adjust dose as needed to a maximum of 200 mcg per kg per minute. Additional loading doses may be administered Perioperative tachycardia and hypertension (2.2) Loading dose: 500 mcg per kg over 1 minute for gradual control (1 mg per kg over 30 seconds for immediate control) Then 50 mcg per kg per min for gradual control (150 mcg per kg per minute for immediate control) adjusted to a maximum of 200 (tachycardia) or 300 (hypertension) mcg per kg per min (2.2) Image1.jpg Image2.jpg
