Drug Catalog - Product Detail
ESTRADIOL TRANSDERMAL SYSTEM 0.1MG/DAY PATCH 8
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0997-08 | AMNEAL PHARMACEUTICALS | 8 | 0.1MG/24HR | TRANSDERMAL SYSTEM |
PACKAGE FILES








Generic Name
ESTRADIOL
Substance Name
ESTRADIOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
TRANSDERMAL
Application Number
ANDA211293
Description
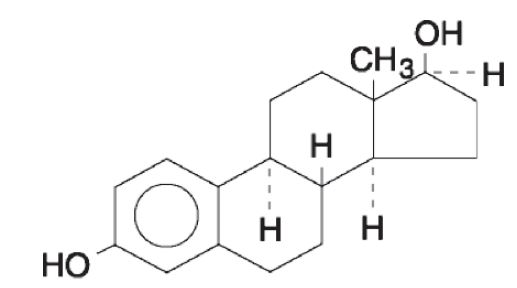
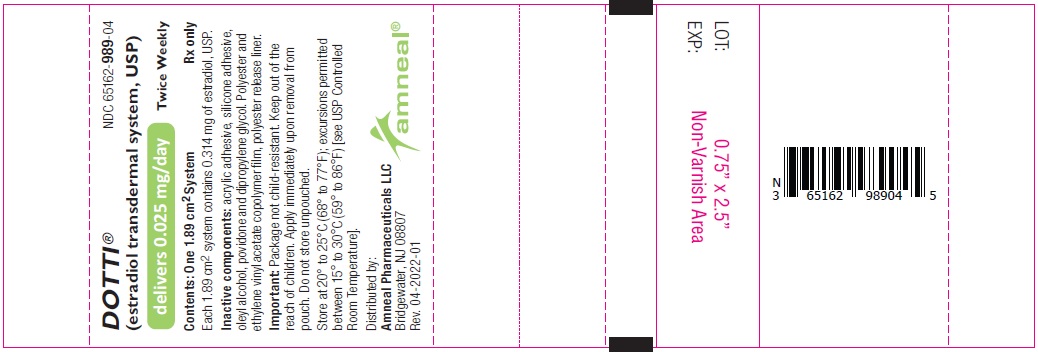
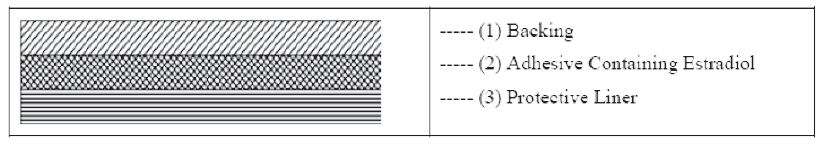
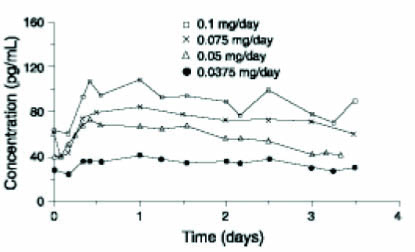
11 DESCRIPTION DOTTI (estradiol transdermal system, USP) contains estradiol, USP in a multipolymeric adhesive. The system is designed to release estradiol, USP continuously upon application to intact skin. Five dosage strengths of DOTTI are available to provide nominal in vivo delivery rates of 0.025, 0.0375, 0.05, 0.075, or 0.1 mg of estradiol, USP per day via the skin. Each corresponding system has an active surface area of 1.89, 2.83, 3.78, 5.66, or 7.55 cm 2 and contains 0.314, 0.470, 0.627, 0.940, or 1.253 mg of estradiol USP, respectively. The composition of the systems per unit area is identical. Estradiol, USP is a white to practically white powder, chemically described as estra-1,3,5 (10)- triene-3,17β-diol. The structural formula is: The molecular formula of estradiol, USP is C 18 H 24 0 2 . The molecular weight is 272.39 g/mol. DOTTI is comprised of 3 layers. Proceeding from the visible surface toward the surface attached to the skin, these layers are (1) polyester and ethylene vinyl acetate copolymer film (2) an adhesive formulation containing estradiol USP, acrylic adhesive, silicone adhesive, oleyl alcohol, NF, povidone, USP and dipropylene glycol, and (3) a polyester release liner which is attached to the adhesive surface and must be removed before the system can be used. The active component of the system is estradiol, USP. The remaining components of the system are pharmacologically inactive. FDA approved acceptance criteria for dissolution test specifications differ from USP. 1 2
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied DOTTI (estradiol transdermal system, USP), 0.025 mg per day -each 1.89 cm 2 system contains 0.314 mg of estradiol, USP for nominal* delivery of 0.025 mg of estradiol, USP per day. Patient Calendar Pack of 8 Systems………………………………….NDC 65162-989-08 DOTTI (estradiol transdermal system, USP), 0.0375 mg per day -each 2.83 cm 2 system contains 0.470 mg of estradiol, USP for nominal* delivery of 0.0375 mg of estradiol, USP per day. Patient Calendar Pack of 8 Systems………………………………….NDC 65162-992-08 DOTTI (estradiol transdermal system, USP), 0.05 mg per day -each 3.78 cm 2 system contains 0.627 mg of estradiol, USP for nominal* delivery of 0.05 mg of estradiol, USP per day. Patient Calendar Pack of 8 Systems………………………………….NDC 65162-993-08 DOTTI (estradiol transdermal system, USP), 0.075 mg per day -each 5.66 cm 2 system contains 0.940 mg of estradiol, USP for nominal* delivery of 0.075 mg of estradiol, USP per day. Patient Calendar Pack of 8 Systems………………………………….NDC 65162-995-08 DOTTI (estradiol transdermal system, USP), 0.1 mg per day -each 7.55 cm 2 system contains 1.253 mg of estradiol, USP for nominal* delivery of 0.1 mg of estradiol, USP per day. Patient Calendar Pack of 8 Systems………………………………….NDC 65162-997-08 [*see DESCRIPTION (11) ] 16.2 Storage and Handling Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Do not store unpouched. Apply immediately upon removal from the protective pouch. Used transdermal systems still contain active hormone. To discard, fold the sticky side of the transdermal system together, place it in a sturdy child-proof container, and place this container in the trash. Used transdermal systems should not be flushed in the toilet.
Indications & Usage
1 INDICATIONS AND USAGE DOTTI is indicated for: DOTTI is an estrogen indicated for: Treatment of moderate to severe vasomotor symptoms due to menopause (1.1) Treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause (1.2) Limitations of Use When prescribing solely for the treatment of moderate to severe vaginal atrophy, first consider the use of topical vaginal products. Treatment of hypoestrogenism due to hypogonadism, castration, or primary ovarian failure (1.3) Prevention of postmenopausal osteoporosis (1.4) Limitations of Use When prescribing solely for the prevention of postmenopausal osteoporosis, first consider the use of non-estrogen medications. Consider estrogen therapy only for women at significant risk of osteoporosis. 1.1 Treatment of Moderate to Severe Vasomotor Symptoms Due to Menopause 1.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy Due to Menopause Limitations of Use : When prescribing solely for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy, first consider the use of topical vaginal products. 1.3 Treatment of Hypoestrogenism Due to Hypogonadism, Castration, or Primary Ovarian Failure 1.4 Prevention of Postmenopausal Osteoporosis Limitations of Use : When prescribing solely for the prevention of postmenopausal osteoporosis, first consider the use of non-estrogen medications. Consider estrogen therapy only for women at significant risk of osteoporosis.
Dosage and Administration
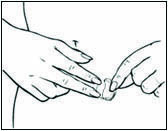
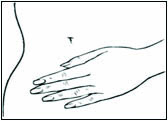
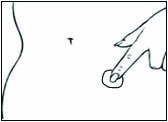
2 DOSAGE AND ADMINISTRATION Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, consider addition of a progestogen to reduce the risk of endometrial cancer. Generally, a woman without a uterus does not need to use a progestogen in addition to her estrogen therapy. In some cases, however, hysterectomized women who have a history of endometriosis may need a progestogen [see Warnings and Precautions (5.2 , 5.14) ] . Use estrogen-alone or in combination with a progestogen at the lowest effective dose and the shortest duration consistent with treatment goals and risks for the individual woman. Reevaluate postmenopausal women periodically as clinically appropriate to determine whether treatment is still necessary. Start therapy with DOTTI 0.0375 mg/day applied to the skin twice weekly for the treatment of moderate to severe vasomotor symptoms due to menopause or moderate to severe symptoms of vulvar and vaginal atrophy symptoms due to menopause. Dosage adjustment should be guided by the clinical response (2.1 , 2.2 , 2.3) Start therapy with DOTTI 0.025 mg/day for the prevention of postmenopausal osteoporosis (2.4) Place DOTTI on a clean, dry area of the lower abdomen or buttocks. Do not apply DOTTI to the breasts (2.5) 2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause Start therapy with DOTTI 0.0375 mg per day applied to the skin twice weekly. Make dosage adjustments based on the clinical response. Initiate DOTTI at once in a woman not currently taking oral estrogens or in a woman switching from another estradiol transdermal therapy. In women who are currently taking oral estrogens, initiate treatment with DOTTI 1 week after withdrawal of oral hormone therapy, or sooner if menopausal symptoms reappear in less than 1 week. Attempts to taper or discontinue DOTTI at 3 to 6 month intervals. Give DOTTI continuously in a woman who does not have an intact uterus. In a woman with an intact uterus, give DOTTI on a cyclic schedule (for example, 3 weeks on DOTTI followed by 1 week off DOTTI). 2.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause Start therapy with DOTTI 0.0375 mg per day applied to the skin twice weekly. Dosage adjustment should be guided by the clinical response. Attempts to taper or discontinue DOTTI at 3 to 6 month intervals. In women not currently taking oral estrogens or in women switching from another estradiol transdermal therapy, treatment with DOTTI may be initiated at once. In women who are currently taking oral estrogens, initiate treatment with DOTTI 1 week after withdrawal of oral hormone therapy, or sooner if menopausal symptoms reappear in less than 1 week. Give DOTTI continuously in a woman who does not have an intact uterus. In a woman with an intact uterus, give DOTTI on a cyclic schedule (for example, 3 weeks on DOTTI followed by 1 week off DOTTI). 2.3 Hypoestrogenism Due to Hypogonadism, Castration, or Primary Ovarian Failure 2.4 Prevention of Postmenopausal Osteoporosis Start therapy with DOTTI 0.025 mg per day applied to the skin twice weekly. In women not currently taking oral estrogens or in women switching from another estradiol transdermal therapy, treatment with DOTTI may be initiated at once. In women who are currently taking oral estrogens, initiate treatment with DOTTI 1 week after withdrawal of oral hormone therapy, or sooner if menopausal symptoms reappear in less than 1 week. DOTTI may be given continuously in a woman who does not have an intact uterus. In a woman with an intact uterus, DOTTI may be given on a cyclic schedule (for example, 3 weeks on DOTTI followed by 1 week off DOTTI). 2.5 Application Instructions Place the adhesive side of DOTTI on a clean, dry area of the trunk of the body (including the abdomen or buttocks). Do not apply DOTTI to the breasts. Replace DOTTI twice weekly. Rotate the sites of application, with an interval of at least 1 week allowed between applications to a particular site. Select an area that is not oily, damaged, or irritated. Avoid the waistline, since tight clothing may rub the system off. Apply the system immediately after opening the pouch and removing the protective liner. Press the system firmly in place with the palm of the hand for about 10 seconds, making sure there is good contact, especially around the edges. In the event that a system falls off, reapply the same system or apply a new system to another location. In either case, continue the original treatment schedule. If a woman has forgotten to apply DOTTI, have her apply a new system as soon as possible. Apply the new system on the original treatment schedule. The interruption of treatment in women taking DOTTI might increase the likelihood of breakthrough bleeding, spotting and recurrence of symptoms.
