Drug Catalog - Product Detail
ESTRADIOL TRANSDERMAL SYSTEM PATCH 0.0375MG/DAY PATCH 4
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00781-7122-54 | SANDOZ | 4 | 0.0375MG/24HR | TRANSDERMAL SYSTEM |
PACKAGE FILES



















Generic Name
ESTRADIOL
Substance Name
ESTRADIOL
Product Type
HUMAN PRESCRIPTION DRUG
Route
TRANSDERMAL
Application Number
NDA020375
Description
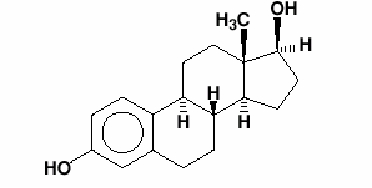
11 DESCRIPTION The Estradiol Transdermal System (estradiol transdermal system), is designed to release estradiol continuously upon application to intact skin. Six (6.5, 9.375, 12.5, 15, 18.75 and 25 cm 2 ) systems are available to provide nominal in vivo delivery of 0.025, 0.0375, 0.05, 0.06, 0.075 or 0.1 mg respectively of estradiol per day. The period of use is 7 days. Each system has a contact surface area of either 6.5, 9.375, 12.5, 15, 18.75 or 25 cm 2 , and contains 2, 2.85, 3.8, 4.55, 5.7 or 7.6 mg of estradiol USP respectively. The composition of the systems per unit area is identical. Estradiol USP is a white, crystalline powder, chemically described as estra-1,3,5(10)-triene-3, 17β-diol. It has an empirical formula of C 18 H 24 O 2 and molecular weight of 272.38. The structural formula is: The Estradiol Transdermal System transdermal system comprises three layers. Proceeding from the visible surface toward the surface attached to the skin, these layers are: 1. A translucent polyethylene film. 2. An acrylate adhesive matrix containing estradiol USP. 3. A protective liner of siliconized or fluoropolymer-coated polyester film is attached to the adhesive surface and must be removed before the system can be used. The active component of the transdermal system is estradiol. The remaining components of the transdermal system (acrylate copolymer adhesive, fatty acid esters, and polyethylene backing) are pharmacologically inactive. Chemical Structure Patch Diagram
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Estradiol Transdermal System, 0.025 mg/day — each 6.5 cm 2 system contains 2 mg of estradiol USP Individual Carton of 4 systems NDC 0781-7119-54 Estradiol Transdermal System, 0.0375 mg/day — each 9.375 cm 2 system contains 2.85 mg of estradiol USP Individual Carton of 4 systems NDC 0781-7122-54 Estradiol Transdermal System, 0.05 mg/day — each 12.5 cm 2 system contains 3.8 mg of estradiol USP Individual Carton of 4 systems ………………NDC 0781-7133-54 Estradiol Transdermal System, 0.06 mg/day — each 15 cm 2 system contains 4.55 mg of estradiol USP Individual Carton of 4 systems NDC 0781-7134-54 Estradiol Transdermal System, 0.075 mg/day — each 18.75 cm 2 system contains 5.7 mg of estradiol USP Individual Carton of 4 systems……………… NDC 0781-7136-54 Estradiol Transdermal System, 0.1 mg/day — each 25 cm 2 system contains 7.6 mg of estradiol USP Individual Carton of 4 systems ……………….NDC 0781-7104-54 16.2 Storage and Handling Store at 20°C to 25°C (66°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). Do not store above 86°F (30°C). Do not store unpouched. Apply immediately upon removal from the protective pouch. Used transdermal systems still contain active hormone. To discard, fold the sticky side of the transdermal system together, place it in a sturdy child-proof container, and place this container in the trash. Used transdermal systems should not be flushed in the toilet.
Indications & Usage
1 INDICATIONS AND USAGE The Estradiol Transdermal System is indicated for: 1.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause [Enter Generic Section here] 1.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause Limitation of Use When prescribing solely for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause, first consider the use of topical vaginal products. 1.3 Treatment of Hypoestrogenism due to Hypogonadism, Castration, or Primary Ovarian Failure [Enter Generic Section here] 1.4 Prevention of Postmenopausal Osteoporosis Limitation of Use When prescribing solely for the prevention of postmenopausal osteoporosis, first consider the use of non-estrogen medications. Consider estrogen therapy only for women at significant risk of osteoporosis
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, consider addition of a progestogen to reduce the risk of endometrial cancer. Generally, a woman without a uterus does not need to use a progestogen in addition to her estrogen therapy. In some cases, however, hysterectomized women who have a history of endometriosis may need a progestogen [see Warnings and Precautions ( 5.2 , 5.14 )] . Use estrogen-alone, or in combination with a progestogen at the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Reevaluate postmenopausal women periodically as clinically appropriate to determine if treatment is still necessary. • Start therapy with the Estradiol Transdermal System 0.025 mg per day applied to the skin once-weekly. Dosage adjustment should be guided by the clinical response ( 2.1 ) • Place the Estradiol Transdermal System on a clean, dry area of the lower abdomen (below the umbilicus) or upper quadrant of the buttock. Do not apply the Estradiol Transdermal System to the breasts ( 2.5 ) 2.1 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause Start therapy with Estradiol Transdermal System 0.025 mg per day applied to the skin once weekly. Make dosage adjustments based on the clinical response. Attempt to taper or discontinue Estradiol Transdermal System at 3 to 6 month intervals. 2.2 Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause Start therapy with Estradiol Transdermal System 0.025 mg per day applied to the skin once weekly. Make dosage adjustments based on the clinical response. Attempt to taper or discontinue Estradiol Transdermal System at 3 to 6 month intervals. 2.3 Treatment of Hypoestrogenism due to Hypogonadism, Castration, or Primary Ovarian Failure Start therapy with 0.025 mg per day applied to the skin once weekly. Make dose adjustment based on the clinical response. 2.4 Prevention of Postmenopausal Osteoporosis Start therapy with Estradiol Transdermal System 0.025 mg per day applied to the skin once weekly. 2.5 Application of the Estradiol Transdermal System Transdermal System Site Selection • Place the adhesive side of Estradiol Transdermal System on a clean, dry area of the lower abdomen or the upper quadrant of the buttock. • Do not apply Estradiol Transdermal System to or near the breasts. • Rotate the sites of application, with an interval of at least 1-week allowed between applications to the same site. • Select an area that is not oily, damaged, or irritated. Avoid the waistline, since tight clothing may rub the transdermal system off. • Avoid application to areas where sitting would dislodge Estradiol Transdermal System. Application • Apply Estradiol Transdermal System immediately after opening the pouch and removing the protective liner. • Press Estradiol Transdermal System firmly in place with the fingers for at least 10 seconds, making sure there is good contact, especially around the edges. • If the system lifts, apply pressure to maintain adhesion. • In the event that a system falls off, reapply it to a different location. If the old system cannot be reapplied, apply a new system for the remainder of the 7-day dosing interval. • Wear only one system at any one time during the 7-day dosing interval. • Swimming, bathing, or using a sauna while using Estradiol Transdermal System has not been studied, and these activities may decrease the adhesion of the system and the delivery of estradiol. 2.6 Removal of the Estradiol Transdermal System Transdermal System • Remove Estradiol Transdermal System carefully and slowly to avoid irritation of the skin. • If any adhesive remains on the skin after removal of Estradiol Transdermal System, allow the area to dry for 15 minutes and then gently rub the area with an oil-based cream or lotion to remove the adhesive residue. • Used patches still contain some active hormones. Carefully fold each patch in half so that it sticks to itself before throwing it away.
