Drug Catalog - Product Detail
ETOPOSIDE FOR INJECTION 20MG/ML 1x5ML
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 16729-0114-31 | ACCORD HEALTHCARE | 5 | 100MG/5ML | SOLUTION |
PACKAGE FILES


Generic Name
ETOPOSIDE
Substance Name
ETOPOSIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
INTRAVENOUS
Application Number
ANDA074513
Description
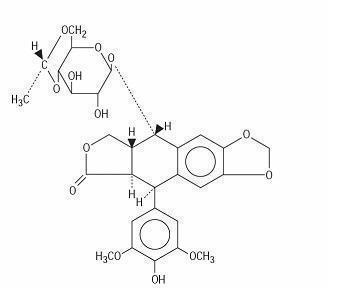
DESCRIPTION Etoposide (also commonly known as VP-16) is a semisynthetic derivative of podophyllotoxin used in the treatment of certain neoplastic diseases. It is 4'-demethylepipodophyllotoxin 9-[4,6-O-(R)-ethylidene-β-D-glucopyranoside]. It is very soluble in methanol and chloroform, slightly soluble in ethanol and sparingly soluble in water and ether. It is made more miscible with water by means of organic solvents. It has a molecular weight of 588.58 and a molecular formula of C 29 H 32 O 13 . Etoposide Injection USP is available for intravenous use as 20 mg/mL solution in 100 mg (5 mL), 250 mg (12.5 mL), 500 mg (25 mL), and 1 g (50 mL) sterile, multiple-dose vials. The pH of the clear, colorless to pale yellow liquid is 3 to 4. Each mL contains 20 mg etoposide USP, 2 mg anhydrous citric acid, 30 mg benzyl alcohol, 80 mg polysorbate 80/tween 80, 650 mg polyethylene glycol 300, and 30.5 percent (v/v) dehydrated alcohol. Vial head space contains nitrogen. The structural formula is: structural formula
How Supplied
HOW SUPPLIED Etoposide Injection USP, 20 mg/mL, is supplied as follows: NDC 16729-114-31 100 mg/5 mL Sterile, Multiple Dose Vial. NDC 16729-114-32 250 mg/12.5 mL Sterile, Multiple Dose Vial NDC 16729-114-08 500 mg/25 mL Sterile, Multiple Dose Vial. NDC 16729-114-11, 1 g/50 mL Sterile, Multiple Dose Vial. Store between 20° to 25°C (68° to 77°F). See USP controlled room temperature.
Indications & Usage
INDICATIONS AND USAGE Etoposide Injection USP is indicated in the management of the following neoplasms: Refractory Testicular Tumors Etoposide Injection USP in combination therapy with other approved chemotherapeutic agents in patients with refractory testicular tumors who have already received appropriate surgical, chemotherapeutic, and radiotherapeutic therapy. Small Cell Lung Cancer Etoposide Injection USP in combination with other approved chemotherapeutic agents as first line treatment in patients with small cell lung cancer.
Dosage and Administration
DOSAGE AND ADMINISTRATION Note: Plastic devices made of acrylic or ABS (a polymer composed of acrylonitrile, butadiene, and styrene) have been reported to crack and leak when used with undiluted Etoposide Injection USP. The usual dose of Etoposide Injection USP in testicular cancer in combination with other approved chemotherapeutic agents ranges from 50 to 100 mg/m 2 /day on days 1 through 5 to 100 mg/m 2 /day on days 1, 3, and 5. In small cell lung cancer, the Etoposide Injection USP dose in combination with other approved chemotherapeutic drugs ranges from 35 mg/m 2 /day for 4 days to 50 mg/m 2 /day for 5 days. For recommended dosing adjustments in patients with renal impairment see PRECAUTIONS . Chemotherapy courses are repeated at 3- to 4-week intervals after adequate recovery from any toxicity. The dosage should be modified to take into account the myelosuppressive effects of other drugs in the combination or the effects of prior X-ray therapy or chemotherapy which may have compromised bone marrow reserve. Administration Precautions As with other potentially toxic compounds, caution should be exercised in handling and preparing the solution of etoposide. Skin reactions associated with accidental exposure to Etoposide Injection USP may occur. The use of gloves is recommended. If etoposide solution contacts the skin or mucosa, immediately and thoroughly wash the skin with soap and water and flush the mucosa with water. Preparation for Intravenous Administration Etoposide Injection USP must be diluted prior to use with either 5% Dextrose Injection, or 0.9% Sodium Chloride Injection, to give a final concentration of 0.2 to 0.4 mg/mL. If solutions are prepared at concentrations above 0.4 mg/mL, precipitation may occur. Hypotension following rapid intravenous administration has been reported; hence, it is recommended that the etoposide Injection USP solution be administered over a 30- to 60-minute period. A longer duration of administration may be used if the volume of fluid to be infused is a concern. Etoposide Injection USP should not be given by rapid intravenous injection. Parenteral drug products should be inspected visually for particulate matter and discoloration (see DESCRIPTION ) prior to administration whenever solution and container permit. Stability Unopened vials of Etoposide Injection USP are stable for 24 months at room temperature (25°C). Vials diluted as recommended to a concentration of 0.2 or 0.4 mg/mL are stable for 96 and 24 hours, respectively, at room temperature (25°C) under normal room fluorescent light in both glass and plastic containers. Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published. 1–7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
