Drug Catalog - Product Detail
FAMOTID TAB 40MG CARL 1000
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 61442-0122-10 | CARLSBAD TECHNOLOGIES | 1000 | 40MG | TABLET |
PACKAGE FILES


Generic Name
Substance Name
Product Type
Route
Application Number
Description
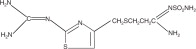
DESCRIPTION The active ingredient in famotidine, is a histamine H 2 -receptor antagonist. Famotidine is N' -(aminosulfonyl)-3-[[[2-[(diamino-methylene)amino]-4- thiazolyl]methyl]thio]propanimidamide. The empirical formula of famotidine is C 8 H 15 N 7 O 2 S 3 and its molecular weight is 337.45. Its structural formula is: Famotidine is a white to pale yellow crystalline compound that is freely soluble in glacial acetic acid, slightly soluble in methanol, very slightly soluble in water, and practically insoluble in ethanol. Each tablet for oral administration contains either 20 mg or 40 mg of famotidine and the following inactive ingredients: hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycolate, sodium starch glycolate, modified corn starch (pregelatinized starch), talc, triacetin, titanium dioxide. Structure
How Supplied
HOW SUPPLIED Famotidine Tablets USP (white round tablets) containing 20mg of famotidine and engraved with . Bottle of 30 (NDC 61442-121-30) Bottle of 100 (NDC 61442-121-01) Bottle of 500 (NDC 61442-121-05) Bottle of 1,000 (NDC 61442-121-10) Famotidine Tablets USP (white round tablets) containing 40mg of famotidine and engraved with . Bottle of 30 (NDC 61442-122-30) Bottle of 100 (NDC 61442-122-01) Bottle of 500 (NDC 61442-122-05) Bottle of 1,000 (NDC 61442-122-10) tab id img 01 tab img 02 STORAGE Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Manufactured and Distributed by: Carlsbad Technology, Inc. Carlsbad, CA 92008 Revised: 06/12 CTI-12 Rev. C
Indications & Usage
INDICATIONS AND USAGE Famotidine is indicated in: Short term treatment of active duodenal ulcer. Most adult patients heal within 4 weeks; there is rarely reason to use famotidine at full dosage for longer than 6 to 8 weeks. Studies have not assessed the safety of famotidine in uncomplicated active duodenal ulcer for periods of more than eight weeks. Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of an active ulcer. Controlled studies in adults have not extended beyond one year. Short term treatment of active benign gastric ulcer. Most adult patients heal within 6 weeks. Studies have not assessed the safety or efficacy of famotidine in uncomplicated active benign gastric ulcer for periods of more than 8 weeks. Short term treatment of gastroesophageal reflux disease (GERD). Famotidine is indicated for short term treatment of patients with symptoms of GERD (see CLINICAL PHARMACOLOGY IN ADULTS , Clinical Studies ). Famotidine is also indicated for the short term treatment of esophagitis due to GERD including erosive or ulcerative disease diagnosed by endoscopy (see CLINICAL PHARMACOLOGY IN ADULTS , Clinical Studies ). Treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison Syndrome, multiple endocrine adenomas) (see CLINICAL PHARMACOLOGY IN ADULTS , Clinical Studies ).
Dosage and Administration
DOSAGE AND ADMINISTRATION Duodenal Ulcer Acute Therapy: The recommended adult oral dosage for active duodenal ulcer is 40 mg once a day at bedtime. Most patients heal within 4 weeks; there is rarely reason to use famotidine at full dosage for longer than 6 to 8 weeks. A regimen of 20 mg b.i.d. is also effective. Maintenance Therapy: The recommended adult oral dose is 20 mg once a day at bedtime. Benign Gastric Ulcer Acute Therapy: The recommended adult oral dosage for active benign gastric ulcer is 40 mg once a day at bedtime. Gastroesophageal Reflux Disease (GERD) The recommended oral dosage for treatment of adult patients with symptoms of GERD is 20 mg b.i.d. for up to 6 weeks. The recommended oral dosage for the treatment of adult patients with esophagitis including erosions and ulcerations and accompanying symptoms due to GERD is 20 or 40 mg b.i.d. for up to 12 weeks (see CLINICAL PHARMACOLOGY IN ADULTS , Clinical Studies ). Dosage for Pediatric Patients <1 year of age Gastroesophageal Reflux Disease (GERD) See PRECAUTIONS , Pediatric Patients <1 year of age . The studies described in PRECAUTIONS , Pediatric Patients <1 year of age suggest the following starting doses in pediatric patients <1 year of age: Gastroesophageal Reflux Disease (GERD) - 0.5 mg/kg/dose of famotidine oral suspension for the treatment of GERD for up to 8 weeks once daily in patients <3 months of age and 0.5 mg/kg/dose twice daily in patients 3 months to <1 year of age. Patients should also be receiving conservative measures (e.g., thickened feedings). The use of intravenous famotidine in pediatric patients <1 year of age with GERD has not been adequately studied. Dosage for Pediatric Patients 1-16 years of age See PRECAUTIONS , Pediatric Patients 1-16 years of age . The studies described in PRECAUTIONS , Pediatric Patients 1-16 years of age suggest the following starting doses in pediatric patients 1-16 years of age: Peptic ulcer — 0.5 mg/kg/day p.o. at bedtime or divided b.i.d. up to 40 mg/day. Gastroesophageal Reflux Disease with or without esophagitis including erosions and ulcerations — 1.0 mg/kg/day p.o. divided b.i.d. up to 40 mg b.i.d. While published uncontrolled studies suggest effectiveness of famotidine in the treatment of gastroesophageal reflux disease and peptic ulcer, data in pediatric patients are insufficient to establish percent response with dose and duration of therapy. Therefore, treatment duration (initially based on adult duration recommendations) and dose should be individualized based on clinical response and/or pH determination (gastric or esophageal) and endoscopy. Published uncontrolled clinical studies in pediatric patients 1-16 years of age have employed doses up to 1 mg/kg/day for peptic ulcer and 2 mg/kg/day for GERD with or without esophagitis including erosions and ulcerations. Pathological Hypersecretory Conditions (e.g., Zollinger-Ellison Syndrome, Multiple Endocrine Adenomas) The dosage of famotidine in patients with pathological hypersecretory conditions varies with the individual patient. The recommended adult oral starting dose for pathological hypersecretory conditions is 20 mg q 6 h. In some patients, a higher starting dose may be required. Doses should be adjusted to individual patient needs and should continue as long as clinically indicated. Doses up to 160 mg q 6 h have been administered to some adult patients with severe Zollinger-Ellison Syndrome. Concomitant Use of Antacids Antacids may be given concomitantly if needed. Dosage Adjustment for Patients with Moderate or Severe Renal Insufficiency In adult patients with moderate (creatinine clearance <50 mL/min) or severe (creatinine clearance <10 mL/min) renal insufficiency, the elimination half-life of famotidine is increased. For patients with severe renal insufficiency, it may exceed 20 hours, reaching approximately 24 hours in anuric patients. Since CNS adverse effects have been reported in patients with moderate and severe renal insufficiency, to avoid excess accumulation of the drug in patients with moderate or severe renal insufficiency, the dose of famotidine may be reduced to half the dose or the dosing interval may be prolonged to 36-48 hours as indicated by the patient's clinical response. Based on the comparison of pharmacokinetic parameters for famotidine in adults and pediatric patients, dosage adjustment in pediatric patients with moderate or severe renal insufficiency should be considered.
