Drug Catalog - Product Detail
FELBAMATE TB 600MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0735-09 | AMNEAL PHARMACEUTICALS | 90 | 600MG | TABLET |
PACKAGE FILES
Generic Name
FELBAMATE
Substance Name
FELBAMATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA201680
Description
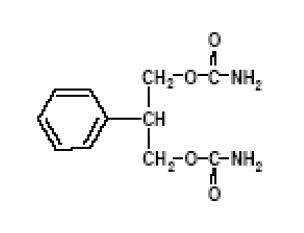
DESCRIPTION Felbamate, USP is an antiepileptic available as 400 mg and 600 mg tablets for oral administration. Its chemical name is 2-phenyl-1,3-propanediol dicarbamate. Felbamate, USP is a white to off-white crystalline powder with a characteristic odor. It is very slightly soluble in water, slightly soluble in ethanol, sparingly soluble in methanol, and freely soluble in dimethyl sulfoxide. The molecular weight is 238.24; felbamate's molecular formula is C 11 H 14 N 2 O 4 ; its structural formula is: The inactive ingredients for felbamate tablets, USP 400 mg and 600 mg are croscarmellose sodium, FD&C Yellow No. 6, D&C Yellow No. 10 and FD&C Red No. 40 (600 mg tablets only), lactose monohydrate, magnesium stearate, microcrystalline cellulose and pregelatinized starch. 95dbc5f9-figure-01
How Supplied
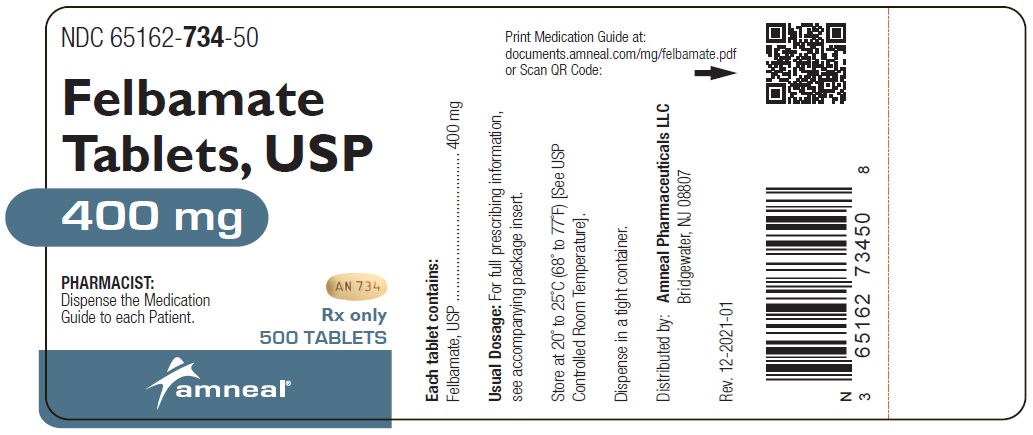
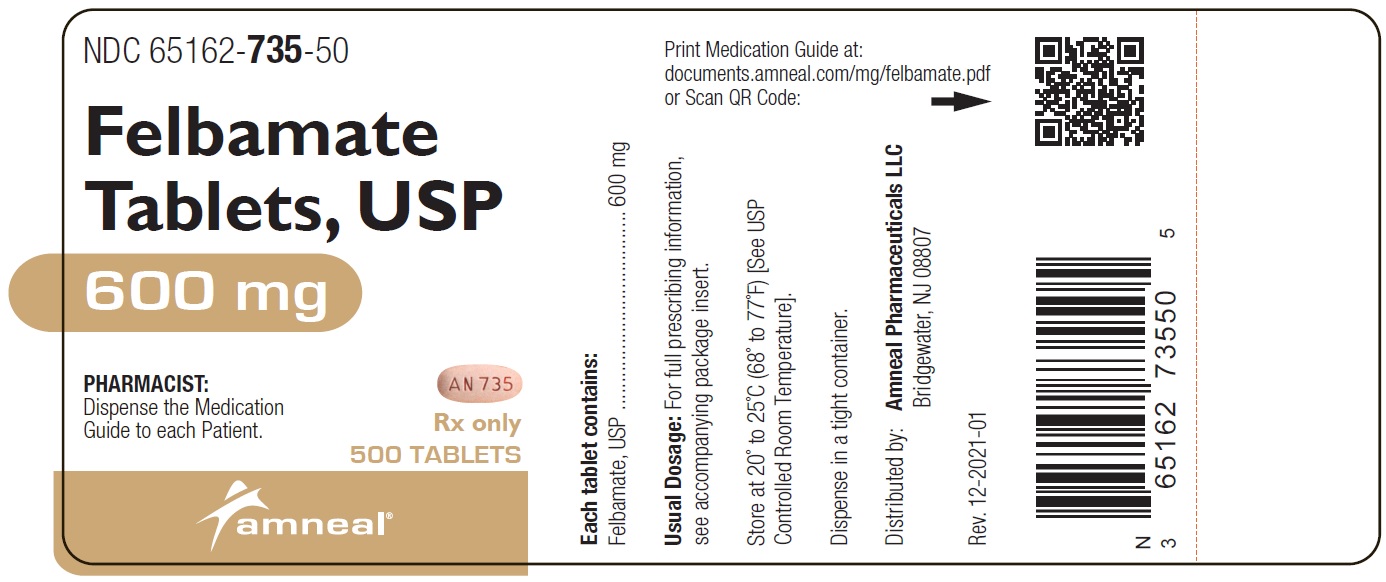
HOW SUPPLIED Felbamate Tablets, USP, 400 mg , are yellow, oval shaped, biconvex tablets, with a bisect on one side and “AN 734” on the other side. They are available as follows: Bottles of 30 count: NDC 65162-734-03 Bottles of 90 count: NDC 65162-734-09 Bottles of 270 count: NDC 65162-734-27 Bottles of 500 count: NDC 65162-734-50 Felbamate Tablets, USP, 600 mg , are peach, oval shaped, biconvex tablets, with a bisect on one side and “AN 735” on the other side. They are available as follows: Bottles of 30 count: NDC 65162-735-03 Bottles of 90 count: NDC 65162-735-09 Bottles of 180 count: NDC 65162-735-18 Bottles of 270 count: NDC 65162-735-27 Bottles of 500 count: NDC 65162-735-50 Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in tight container. To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Indications & Usage
INDICATIONS AND USAGE Felbamate tablets, USP are not indicated as a first line antiepileptic treatment (see Warnings ). Felbamate tablets, USP are recommended for use only in those patients who respond inadequately to alternative treatments and whose epilepsy is so severe that a substantial risk of aplastic anemia and/or liver failure is deemed acceptable in light of the benefits conferred by its use. If these criteria are met and the patient has been fully advised of the risk, and has provided written acknowledgment, felbamate tablets, USP can be considered for either monotherapy or adjunctive therapy in the treatment of partial seizures, with and without generalization, in adults with epilepsy and as adjunctive therapy in the treatment of partial and generalized seizures associated with Lennox-Gastaut syndrome in children.
Dosage and Administration
DOSAGE AND ADMINISTRATION Felbamate tablets, USP have been studied as monotherapy and adjunctive therapy in adults and as adjunctive therapy in children with seizures associated with Lennox-Gastaut syndrome. As felbamate tablets, USP are added to or substituted for existing AEDs, it is strongly recommended to reduce the dosage of those AEDs in the range of 20% to 33% to minimize side effects (see Drug Interactions subsection). Dosage Adjustment in the Renally Impaired: Felbamate, USP should be used with caution in patients with renal dysfunction. In the renally impaired, starting and maintenance doses should be reduced by one-half (See CLINICAL PHARMACOLOGY / Pharmacokinetics and PRECAUTIONS ). Adjunctive therapy with medications which affect felbamate, USP plasma concentrations, especially AEDs, may warrant further reductions in felbamate, USP daily doses in patients with renal dysfunction. Adults (14 years of age and over) The majority of patients received 3600 mg/day in clinical trials evaluating its use as both monotherapy and adjunctive therapy. Monotherapy: (Initial therapy) Felbamate tablets, USP have not been systematically evaluated as initial monotherapy. Initiate felbamate tablets, USP at 1200 mg/day in divided doses three or four times daily. The prescriber is advised to titrate previously untreated patients under close clinical supervision, increasing the dosage in 600-mg increments every 2 weeks to 2400 mg/day based on clinical response and thereafter to 3600 mg/day if clinically indicated. Conversion to Monotherapy: Initiate felbamate tablets, USP at 1200 mg/day in divided doses three or four times daily. Reduce the dosage of concomitant AEDs by one-third at initiation of felbamate tablets, USP therapy. At week 2, increase the felbamate tablets, USP dosage to 2400 mg/day while reducing the dosage of other AEDs up to an additional one-third of their original dosage. At week 3, increase the felbamate tablets, USP dosage up to 3600 mg/day and continue to reduce the dosage of other AEDs as clinically indicated. Adjunctive Therapy: Felbamate tablets, USP should be added at 1200 mg/day in divided doses three or four times daily while reducing present AEDs by 20% in order to control plasma concentrations of concurrent phenytoin, valproic acid, phenobarbital and carbamazepine and its metabolites. Further reductions of the concomitant AEDs dosage may be necessary to minimize side effects due to drug interactions. Increase the dosage of felbamate, USP by 1200 mg/day increments at weekly intervals to 3600 mg/day. Most side effects seen during felbamate tablets, USP adjunctive therapy resolve as the dosage of concomitant AEDs is decreased. Table 6 Dosage Table (adults) Dosage reduction of concomitant AEDs WEEK 1 REDUCE original dose by 20% to 33%* WEEK 2 REDUCE original dose by up to an additional 1/3* WEEK 3 REDUCE as clinically indicated Felbamate Tablet, USP Dosage 1200 mg/day Initial dose 2400 mg/day Therapeutic dosage range 3600 mg/day Therapeutic dosage range *See Adjunctive and Conversion to Monotherapy sections. While the above felbamate tablets, USP conversion guidelines may result in a felbamate tablet, USP 3600 mg/day dose within 3 weeks, in some patients titration to a 3600 mg/day felbamate tablets, USP dose has been achieved in as little as 3 days with appropriate adjustment of other AEDs. Children with Lennox-Gastaut Syndrome (Ages 2 to 14 years) Adjunctive Therapy: Felbamate tablets, USP should be added at 15 mg/kg/day in divided doses three or four times daily while reducing present AEDs by 20% in order to control plasma levels of concurrent phenytoin, valproic acid, phenobarbital and carbamazepine and its metabolites. Further reductions of the concomitant AEDs dosage may be necessary to minimize side effects due to drug interactions. Increase the dosage of felbamate tablets, USP by 15 mg/kg/day increments at weekly intervals to 45 mg/kg/day. Most side effects seen during felbamate tablets, USP adjunctive therapy resolve as the dosage of concomitant AEDs is decreased.
