Drug Catalog - Product Detail
FENOFIBRIC ACID DR CP 135MG 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00115-1555-10 | AMNEAL PHARMACEUTICALS | 90 | 135MG | CAPSULE |
PACKAGE FILES



Generic Name
FENOFIBRIC ACID
Substance Name
FENOFIBRIC ACID
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
NDA022224
Description
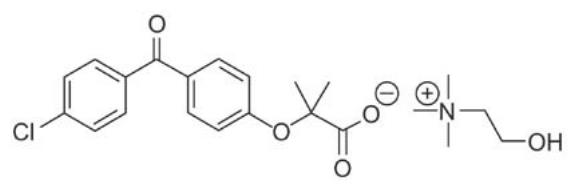
11 DESCRIPTION Fenofibric acid delayed-release capsules (fenofibric acid) are a peroxisome proliferator-activated receptor (PPAR) alpha agonist available as delayed release capsules for oral administration. Each delayed release capsule contains choline fenofibrate, equivalent to 45 mg or 135 mg of fenofibric acid. The chemical name for choline fenofibrate is ethanaminium, 2-hydroxy-N,N,N-trimethyl, 2-{4-(4-chlorobenzoyl)phenoxy] -2-methylpropanoate (1:1) with the following structural formula: The empirical formula is C 22 H 28 ClNO 5 and the molecular weight is 421.91. Choline fenofibrate is freely soluble in water. The melting point is approximately 210°C. Choline fenofibrate is a white to yellow powder, which is stable under ordinary conditions. Each delayed release capsule contains enteric coated mini-tablets comprised of choline fenofibrate and the following inactive ingredients: colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, methacrylic acid copolymer, povidone, sodium stearyl fumarate, talc, triethyl citrate, water. The capsule shell of the 45 mg capsule contains the following inactive ingredients: black iron oxide, gelatin, red iron oxide, titanium dioxide, and yellow iron oxide. The capsule shell of the 135 mg capsule contains the following inactive ingredients: FD&C Blue #2, gelatin, titanium dioxide, and yellow iron oxide. Chemical structure for fenofibric acid.
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Fenofibric acid delayed-release capsules 45 mg: A reddish brown to orange brown cap and a yellow body imprinted in black ink the number “45”, available in bottles of 90 (NDC 0115-1554-10). Fenofibric acid delayed-release capsules 135 mg: A blue cap and a yellow body imprinted in black ink the number “135”, available in bottles of 90 (NDC 0115-1555-10). Store at 25°C (77°F); excursions permitted to 15°-30°C (59° to 86°F) [See USP controlled room temperature]. Keep out of the reach of children. Protect from moisture.
Indications & Usage
1 INDICATIONS AND USAGE Fenofibric acid delayed-release capsules are indicated as adjunctive therapy to diet: • to reduce triglyceride (TG) levels in adults with severe hypertriglyceridemia (TG greater than or equal to 500 mg/dL). • to reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia when use of recommended LDL-C lowering therapy is not possible. Limitations of Use • Markedly elevated levels of serum TG (e.g., > 2,000 mg/dL) may increase the risk of developing pancreatitis. The effect of fenofibrate therapy on reducing this risk has not been determined [see Warnings and Precautions ( 5.7 )] . • Fenofibrate did not reduce coronary heart disease morbidity and mortality in two large, randomized controlled trials of patients with type 2 diabetes mellitus [see Warnings and Precautions ( 5.1 ) and Clinical Studies ( 14.4 )] . Fenofibric acid delayed-release capsules are a peroxisome proliferator-activated receptor (PPAR) alpha agonist indicated as adjunct to diet: to reduce triglyceride (TG) levels in adults with severe hypertriglyceridemia (TG greater than or equal to 500 mg/dL) ( 1 ). to reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia when use of recommended LDL-C lowering therapy is not possible ( 1 ). Limitations of Use: Markedly elevated levels of serum TG (e.g., >2,000 mg/dL) may increase the risk of developing pancreatitis. The effect of fenofibrate therapy on reducing this risk has not been determined ( 1 ). Fenofibrate did not reduce coronary heart disease morbidity and mortality in two large, randomized controlled trials of patients with type 2 diabetes mellitus ( 1 ).
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Severe h ypertriglyceridemia: 45 to 135 mg orally once daily; the dosage should be adjusted according to patient response ( 2.2 ). Primary hyperlipidemia : 135 mg orally once daily ( 2.3 ). Administer as a single dose, at any time of day, with or without food ( 2.2 ). Assess TG when clinically appropriate, as early as 4 to 8 weeks after initiating fenofibric acid delayed-release capsules. Discontinue fenofibric acid delayed-release capsules in patients who do not have an adequate response after 2 months of treatment ( 2.2 ). Swallow capsules whole. Do not crush, break, dissolve, or chew ( 2.2 ). Renal impair ment : Initial dosage of 45 mg orally once daily ( 2.3 ). Geriatric patients: Select the dosage on the basis of renal function ( 2.4 ). 2.1 Prior to Initiation of Fenofibric Acid Delayed-Release Capsules • Assess lipid levels before initiating therapy. Identify other causes (e.g., diabetes mellitus, hypothyroidism, or medications) of high TG levels and manage as appropriate. • Patients should be placed on an appropriate lipid-lowering diet before receiving fenofibric acid delayed-release capsules, and should continue this diet during treatment with fenofibric acid delayed-release capsules. • In patients with diabetes and fasting chylomicronemia, improve glycemic control prior to considering starting Trilipix. 2.2 Recommended Dosage and Administration • Severe hypertriglyceridemia: ○ The recommended dosage of fenofibric acid delayed-release capsules is 45 mg or 135 mg orally once daily. ○ Dosage should be individualized according to patient response, and should be adjusted if necessary following repeat lipid determinations at 4 to 8 week intervals. • Primary hyperlipidemia: ○ The recommended dosage of fenofibric acid delayed-release capsules is 135 mg orally once daily. • Administer fenofibric acid delayed-release capsules as a single dose at any time of day, with or without food. • Advise patients to swallow fenofibric acid delayed-release capsules whole. Do not crush, break, dissolve, or chew capsules. • Assess TG when clinically appropriate, as early as 4 to 8 weeks after initiating fenofibric acid delayed-release capsules. Discontinue fenofibric acid delayed-release capsules in patients who do not have an adequate response after two months of treatment. • If a dose is missed, advise patients not to take an extra dose. Resume treatment with the next dose. • Advise patients to take fenofibric acid delayed-release capsules at least 1 hour before or 4 hours to 6 hours after a bile acid binding resin to avoid impeding its absorption. 2.3 Recommended Dosage in Patients with Renal Impairment • Assess renal function prior to initiation of Trilipix and periodically thereafter [see Warnings and Precautions ( 5.4 )] . • Treatment with fenofibric acid delayed-release capsules should be initiated at a dosage of 45 mg orally once daily in patients with mild to moderately impaired renal function (eGFR 30 to <60 mL/min/1.73m 2 ), and increased only after evaluation of the effects on renal function and TG levels at this dosage. • Fenofibric acid delayed-release capsules are contraindicated in patients with severe renal impairment (eGFR <30 mL/min/1.73m 2 ), including those with end-stage renal disease (ESRD) and those receiving dialysis [see Use in Specific Populations ( 8.6 ) and Clinical Pharmacology ( 12.3 )] . 2.4 Recommended Dosage in Geriatric Patients Dosage selection for geriatric patients should be made on the basis of renal function [see Use in Specific Populations ( 8.6 ) and Clinical Pharmacology ( 12.3 )] .
