Drug Catalog - Product Detail
FINASTER TAB 5MG AURO 90
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0149-90 | AUROBINDO PHARMA | 90 | 5MG | TABLET |
PACKAGE FILES



Generic Name
FINASTERIDE
Substance Name
FINASTERIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078341
Description
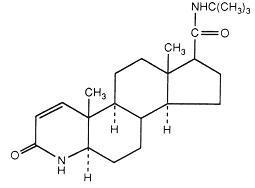
11 DESCRIPTION Finasteride, a synthetic 4-azasteroid compound, is a specific inhibitor of steroid Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into 5α-dihydrotestosterone (DHT). Finasteride is 4-azaandrost-1-ene-17-carboxamide, N-(1,1-dimethylethyl)-3-oxo-,(5α,17β)-. The molecular formula of finasteride is C 23 H 36 N 2 O 2 and its molecular weight is 372.55. Its structural formula is: Finasteride USP is a white crystalline powder with a melting point near 250°C. It is freely soluble in chloroform and in lower alcohol solvents, but is practically insoluble in water. Finasteride tablets, USP for oral administration are film-coated tablets that contain 5 mg of finasteride and the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, sodium starch glycolate, pregelatinised starch (maize), docusate sodium, magnesium stearate, hypromellose, hydroxypropyl cellulose, titanium dioxide, talc, iron oxide yellow, and FD&C Blue #2 aluminum lake. chemical structure
How Supplied
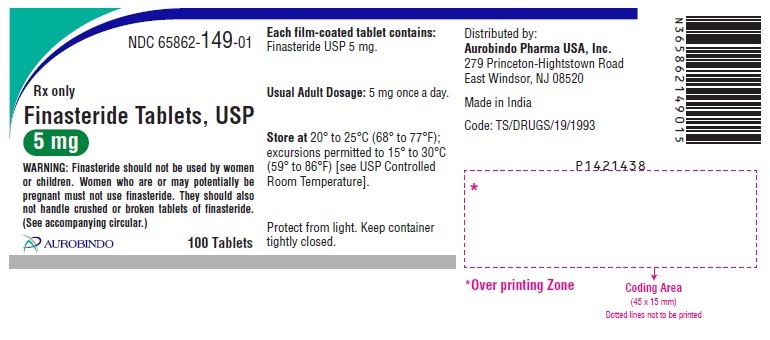
16 HOW SUPPLIED/STORAGE AND HANDLING Finasteride Tablets USP, 5 mg are blue colored, circular, biconvex, beveled edged film-coated tablets debossed with ‘E’ on one side and ‘61’ on the other side. Bottles of 30 NDC 65862-149-30 Bottles of 90 NDC 65862-149-90 Bottles of 100 NDC 65862-149-01 Bottles of 500 NDC 65862-149-05 Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light and keep container tightly closed. Females should not handle crushed or broken finasteride tablets when they are pregnant or may potentially be pregnant because of the possibility of absorption of finasteride and the subsequent potential risk to a male fetus [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1) ].
Indications & Usage
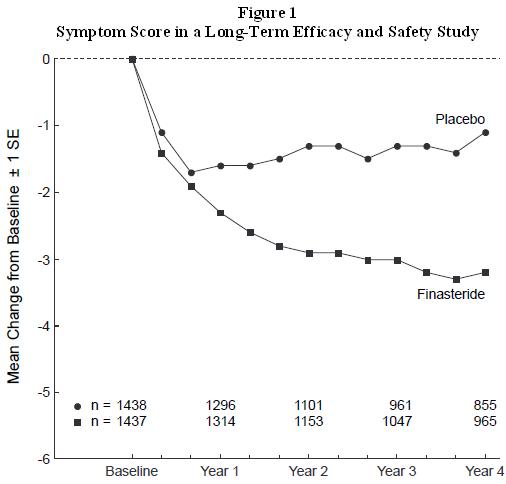
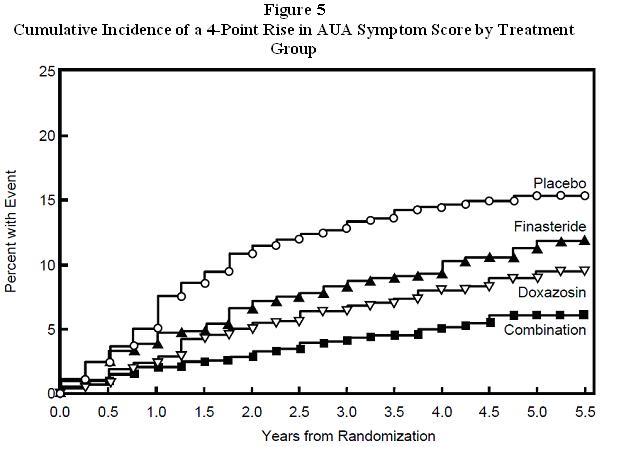
1 INDICATIONS AND USAGE Finasteride, is a 5α-reductase inhibitor, indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to (1.1) : Improve symptoms Reduce the risk of acute urinary retention Reduce the risk of the need for surgery including transurethral resection of the prostate (TURP) and prostatectomy. Finasteride tablets administered in combination with the alpha-blocker doxazosin are indicated to reduce the risk of symptomatic progression of BPH (a confirmed ≥4 point increase in American Urological Association (AUA) symptom score) (1.2) . Limitations of Use: Finasteride tablets are not approved for the prevention of prostate cancer (1.3) . 1.1 Monotherapy Finasteride tablets are indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to: - Improve symptoms - Reduce the risk of acute urinary retention - Reduce the risk of the need for surgery including transurethral resection of the prostate (TURP) and prostatectomy. 1.2 Combination with Alpha-Blocker Finasteride tablets administered in combination with the alpha-blocker doxazosin are indicated to reduce the risk of symptomatic progression of BPH (a confirmed ≥4 point increase in American Urological Association (AUA) symptom score). 1.3 Limitations of Use Finasteride tablets are not approved for the prevention of prostate cancer.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Finasteride tablets may be administered with or without meals. Finasteride tablets may be administered with or without meals (2) . Monotherapy: One tablet (5 mg) taken once a day (2.1) . Combination with Doxazosin: One tablet (5 mg) taken once a day in combination with the alpha-blocker doxazosin (2.2) . 2.1 Monotherapy The recommended dose of finasteride tablets is one tablet (5 mg) taken once a day [see Clinical Studies (14.1) ] . 2.2 Combination with Alpha-Blocker The recommended dose of finasteride tablets is one tablet (5 mg) taken once a day in combination with the alpha-blocker doxazosin [see Clinical Studies (14.2) ].
