Drug Catalog - Product Detail
FLECAINIDE ACETATE TB 100MG 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65162-0642-10 | AMNEAL PHARMACEUTICALS | 100 | 100MG | TABLET |
PACKAGE FILES



Generic Name
FLECAINIDE ACETATE
Substance Name
FLECAINIDE ACETATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA075442
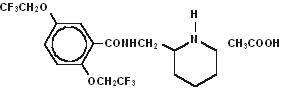
Description
DESCRIPTION Flecainide acetate is an antiarrhythmic drug available in tablets of 50, 100, or 150 mg for oral administration. Flecainide acetate is benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-, monoacetate. The structural formula is given below. Molecular formula: C 17 H 20 F 6 N 2 O 3 •C 2 H 4 O 2 Molecular weight: 474.40 Flecainide acetate, USP is a white crystalline substance with a pK a of 9.3. It has an aqueous solubility of 48.4 mg/mL at 37°C. Flecainide acetate tablets, USP also contain the following inactive ingredients: croscarmellose sodium, microcrystalline cellulose and magnesium stearate. d980fd55-figure-01
How Supplied
HOW SUPPLIED Flecainide acetate tablets, USP are available as follows: 50 mg: White, round tablets debossed with “AN” above “641” on one side and plain on other side. Bottles of 100: NDC 65162-641-10 Bottles of 1000: NDC 65162-641-11 100 mg: White, round tablets debossed with “AN” above “642” with a single-line bisect separating them on one side and plain on other side. Bottles of 100: NDC 65162-642-10 Bottles of 1000: NDC 65162-642-11 150 mg: White, oval tablets debossed with “AN”, “643” with a single-line bisect separating them on one side and plain on other side. Bottles of 100: NDC 65162-643-10 Bottles of 1000: NDC 65162-643-11 Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature] in a tight, light-resistant container. Distributed by: Amneal Pharmaceuticals LLC Bridgewater, NJ 08807 Rev. 05-2018-01
Indications & Usage
INDICATIONS AND USAGE In patients without structural heart disease, flecainide is indicated for the prevention of - paroxysmal supraventricular tachycardias (PSVT), including atrioventricular nodal reentrant tachycardia, atrioventricular reentrant tachycardia and other supraventricular tachycardias of unspecified mechanism associated with disabling symptoms - paroxysmal atrial fibrillation/flutter (PAF) associated with disabling symptoms. Flecainide is also indicated for the prevention of - documented ventricular arrhythmias, such as sustained ventricular tachycardia ( sustained VT), that in the judgment of the physician, are life-threatening. Use of flecainide for the treatment of sustained VT, like other antiarrhythmics, should be initiated in the hospital. The use of flecainide is not recommended in patients with less severe ventricular arrhythmias even if the patients are symptomatic. Because of the proarrhythmic effects of flecainide, its use should be reserved for patients in whom, in the opinion of the physician, the benefits of treatment outweigh the risks. Flecainide should not be used in patients with recent myocardial infarction (see Boxed WARNINGS ). Use of flecainide in chronic atrial fibrillation has not been adequately studied and is not recommended (see Boxed WARNINGS ). As is the case for other antiarrhythmic agents, there is no evidence from controlled trials that the use of flecainide favorably affects survival or the incidence of sudden death.
Dosage and Administration
DOSAGE AND ADMINISTRATION For patients with sustained VT, no matter what their cardiac status, flecainide, like other antiarrhythmics, should be initiated in hospital with rhythm monitoring. Flecainide has a long half-life (12 to 27 hours in patients). Steady-state plasma levels, in patients with normal renal and hepatic function, may not be achieved until the patient has received 3 to 5 days of therapy at a given dose. Therefore, increases in dosage should be made no more frequently than once every four days , since during the first 2 to 3 days of therapy the optimal effect of a given dose may not be achieved. For patients with PSVT and patients with PAF the recommended starting dose is 50 mg every 12 hours. Flecainide acetate doses may be increased in increments of 50 mg bid every four days until efficacy is achieved. For PAF patients, a substantial increase in efficacy without a substantial increase in discontinuations for adverse experiences may be achieved by increasing the flecainide acetate dose from 50 to 100 mg bid. The maximum recommended dose for patients with paroxysmal supraventricular arrhythmias is 300 mg/day. For sustained VT the recommended starting dose is 100 mg every 12 hours. This dose may be increased in increments of 50 mg bid every four days until efficacy is achieved. Most patients with sustained VT do not require more than 150 mg every 12 hours (300 mg/day), and the maximum dose recommended is 400 mg/day. In patients with sustained VT, use of higher initial doses and more rapid dosage adjustments have resulted in an increased incidence of proarrhythmic events and CHF, particularly during the first few days of dosing (see WARNINGS ). Therefore, a loading dose is not recommended. Intravenous lidocaine has been used occasionally with flecainide while awaiting the therapeutic effect of flecainide. No adverse drug interactions were apparent. However, no formal studies have been performed to demonstrate the usefulness of this regimen. An occasional patient not adequately controlled by (or intolerant to) a dose given at 12-hour intervals may be dosed at eight-hour intervals. Once adequate control of the arrhythmia has been achieved, it may be possible in some patients to reduce the dose as necessary to minimize side effects or effects on conduction. In such patients, efficacy at the lower dose should be evaluated. Flecainide should be used cautiously in patients with a history of CHF or myocardial dysfunction (see WARNINGS ). Any use of flecainide in children should be directly supervised by a cardiologist skilled in the treatment of arrhythmias in children. Because of the evolving nature of information in this area, specialized literature should be consulted. Under six months of age, the initial starting dose of flecainide acetate in children is approximately 50 mg/M 2 body surface area daily, divided into two or three equally spaced doses. Over six months of age, the initial starting dose may be increased to 100 mg/M 2 per day. The maximum recommended dose is 200 mg/M 2 per day. This dose should not be exceeded. In some children on higher doses, despite previously low plasma levels, the level has increased rapidly to far above therapeutic values while taking the same dose. Small changes in dose may also lead to disproportionate increases in plasma levels. Plasma trough (less than one hour pre-dose) flecainide levels and electrocardiograms should be obtained at presumed steady state (after at least five doses) either after initiation or change in flecainide dose, whether the dose was increased for lack of effectiveness, or increased growth of the patient. For the first year on therapy, whenever the patient is seen for reasons of clinical follow-up, it is suggested that a 12-lead electrocardiogram and plasma trough flecainide level are obtained. The usual therapeutic level of flecainide in children is 200 to 500 ng/mL. In some cases, levels as high as 800 ng/mL may be required for control. In patients with severe renal impairment (creatinine clearance of 35 mL/min/1.73 square meters or less), the initial dosage should be 100 mg once daily (or 50 mg bid); when used in such patients, frequent plasma level monitoring is required to guide dosage adjustments (see Plasma Level Monitoring ). In patients with less severe renal disease, the initial dosage should be 100 mg every 12 hours; plasma level monitoring may also be useful in these patients during dosage adjustment. In both groups of patients, dosage increases should be made very cautiously when plasma levels have plateaued (after more than four days), observing the patient closely for signs of adverse cardiac effects or other toxicity. It should be borne in mind that in these patients it may take longer than four days before a new steady-state plasma level is reached following a dosage change. Based on theoretical considerations, rather than experimental data, the following suggestion is made: when transferring patients from another antiarrhythmic drug to flecainide allow at least two to four plasma half-lives to elapse for the drug being discontinued before starting flecainide at the usual dosage. In patients where withdrawal of a previous antiarrhythmic agent is likely to produce life-threatening arrhythmias, the physician should consider hospitalizing the patient. When flecainide is given in the presence of amiodarone, reduce the usual flecainide dose by 50% and monitor the patient closely for adverse effects. Plasma level monitoring is strongly recommended to guide dosage with such combination therapy (see below). Plasma Level Monitoring The large majority of patients successfully treated with flecainide were found to have trough plasma levels between 0.2 and 1 mcg/mL. The probability of adverse experiences, especially cardiac, may increase with higher trough plasma levels, especially when these exceed 1 mcg/mL. Periodic monitoring of trough plasma levels may be useful in patient management. Plasma level monitoring is required in patients with severe renal failure or severe hepatic disease, since elimination of flecainide from plasma may be markedly slower. Monitoring of plasma levels is strongly recommended in patients on concurrent amiodarone therapy and may also be helpful in patients with CHF and in patients with moderate renal disease.
