Drug Catalog - Product Detail
FLUOCINONIDE CREAM USP 0.1% 60GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68462-0505-65 | GLENMARK PHARMACEUTICALS | 60 | 0.1% | CREAM |
PACKAGE FILES



Generic Name
FLUOCINONIDE
Substance Name
FLUOCINONIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA091282
Description
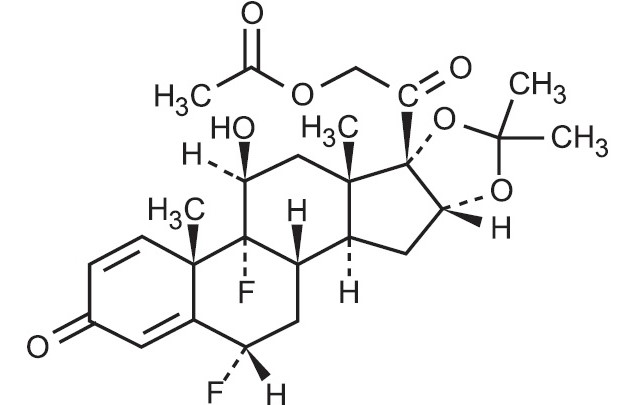
11 DESCRIPTION Fluocinonide cream USP, 0.1% contains fluocinonide USP, a synthetic corticosteroid for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents. Fluocinonide USP has the chemical name 6 alpha, 9 alpha-difluoro-11 beta, 21-dihydroxy-16 alpha, 17 alpha-isopropylidenedioxypregna-1, 4-diene-3,20-dione 21-acetate. Its chemical formula is C 26 H 32 F 2 O 7 and it has a molecular weight of 494.52. It has the following chemical structure: Fluocinonide is a white powder. It is sparingly soluble in acetone and inchloroform; slightly soluble in alcohol, methanol, dioxane, acetonitrile and ethyl acetate; very slightly soluble inether; and practically insoluble in water. Each gram of fluocinonide cream USP, 0.1%contains 1 mg micronized fluocinonide USP in a cream base of propylene glycol, diethylene glycol monoethyl ether, glyceryl stearate (and) PEG-100 stearate, glyceryl monostearate, purified water, carbomer, diisopropanolamine and anhydrous citric acid. structure
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Fluocinonide cream USP, 0.1% is a white cream and is supplied in tubes as follows: 30 g (NDC 68462-505-35) 60 g (NDC 68462-505-65) 120 g (NDC 68462-505-66) Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP controlled room temperature]. Keep the tube tightly closed.
Indications & Usage
1 INDICATIONS AND USAGE Fluocinonide cream USP, 0.1%is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses in patients 12 years of age or older. ( 1 ) Limitation of Use: • Treatment beyond 2 consecutive weeks is not recommended and the total dosage should not exceed 60 g per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. ( 1 ) • Avoid use on the face, groin, or axillae. ( 1.2 ) • Avoid use in perioral dermatitis or rosacea. 1.1 Indication Fluocinonide cream USP, 0.1% is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses in patients 12 years of age or older [ see Use in Specific Populations ( 8.4 ) ]. 1.2 Limitation of Use Treatment beyond 2 consecutive weeks is not recommended and the total dosage should not exceed 60 g per week because the safety of fluocinonide cream USP, 0.1% for longer than 2 weeks has not been established and because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Therapy should be discontinued when control of the disease is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary. Do not use more than half of the 120 g tube per week. Fluocinonide cream USP, 0.1% should not be used in the treatment of rosacea or perioral dermatitis, and should not be used on the face, groin, or axillae.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION For topical use only. Fluocinonide cream USP, 0.1% is not for ophthalmic, oral, or intravaginal use. For psoriasis, apply a thin layer of fluocinonide cream USP, 0.1% once or twice daily to the affected skin areas as directed by a physician. Twice daily application for the treatment of psoriasis has been shown to be more effective in achieving treatment success during 2 weeks of treatment. For atopic dermatitis, apply a thin layer of fluocinonide cream USP, 0.1% once daily to the affected skin areas as directed by a physician. Once daily application for the treatment of atopic dermatitis has been shown to be as effective as twice daily treatment in achieving treatment success during 2 weeks of treatment [ see Clinical Studies ( 14 ) ]. For corticosteroid responsive dermatoses, other than psoriasis or atopic dermatitis, apply a thin layer of fluocinonide cream USP, 0.1% once or twice daily to the affected areas as directed by a physician. For topical use only. Fluocinonide cream USP, 0.1%is not for ophthalmic, oral, or intravaginal use. ( 2 ) Psoriasis: apply a thin layer once or twice daily to the affected skin areas. ( 2 ) Atopic Dermatitis: apply a thin layer once daily to the affected skin areas. ( 2 ) Corticosteroid Responsive Dermatoses, other than psoriasis or atopic dermatitis: apply a thin layer once or twice daily to the affected areas. ( 2 )
