Drug Catalog - Product Detail
FLUOCINONIDE OINTMENT 0.0005 OINT 30GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 47781-0569-73 | ALVOGEN | 30 | 0.05% | OINTMENT |
PACKAGE FILES


Generic Name
FLUOCINONIDE
Substance Name
FLUOCINONIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
NDA016909
Description
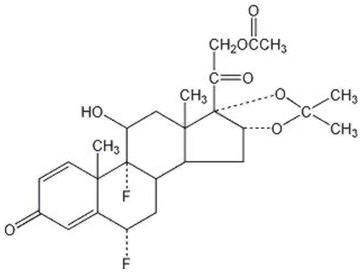
DESCRIPTION Fluocinonide Ointment, 0.05% is intended for topical administration. The active component is the corticosteroid fluocinonide, which is the 21-acetate ester of fluocinolone acetonide and has the chemical name pregna-1,4-diene-3,20-dione,21-(acetyloxy)-6,9-difluoro-11-hydroxy-16,17-[(1-methylethylidene)bis(oxy)]-,(6α,11β,16α)-. It has the following chemical structure: Fluocinonide Ointment contains fluocinonide 0.5 mg/g in a specially formulated ointment base consisting of glyceryl monostearate, white petrolatum, propylene carbonate, propylene glycol and white wax. It provides the occlusive and emollient effects desirable in an ointment. In this formulation, the active ingredient is totally in solution. Chemical Structure
How Supplied
HOW SUPPLIED Fluocinonide Ointment, 0.05% is supplied in 15 g Tube – NDC 47781-569-72 30 g Tube – NDC 47781-569-73 60 g Tube – NDC 47781-569-26 Store at room temperature. Avoid excess heat, above 30°C (86°F)
Indications & Usage
INDICATIONS AND USAGE Fluocinonide Ointment is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
Dosage and Administration
DOSAGE AND ADMINISTRATION Fluocinonide Ointment is generally applied to the affected area as a thin film from two to four times daily depending on the severity of the condition. Occlusive dressings may be used for the management of psoriasis or recalcitrant conditions. If an infection develops, the use of the occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
