Drug Catalog - Product Detail
FLURANDRENOLIDE 0.05% LOTION 120GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0928-03 | PADAGIS | 120 | 0.05% | LOTION |
PACKAGE FILES

Generic Name
FLURANDRENOLIDE
Substance Name
FLURANDRENOLIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA207133
Description
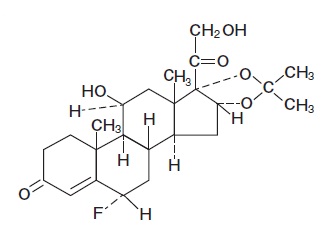
DESCRIPTION Flurandrenolide Lotion USP, 0.05% is a potent corticosteroid intended for topical use. Flurandrenolide occurs as white to off-white, fluffy, crystalline powder and is odorless. Flurandrenolide is practically insoluble in water and in ether. One g dissolves in 72 mL of alcohol and in 10 mL of chloroform. The molecular weight of flurandrenolide is 436.52. The chemical name of flurandrenolide is Pregn-4-ene-3,20-dione, 6-fluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis (oxy)]-, (6α, 11β, 16α)-; its empirical formula is C 24 H 33 FO 6 . The structure is as follows: Each mL of Flurandrenolide Lotion USP, 0.05% contains 0.5 mg (1.145 μmol) (0.05%) flurandrenolide in an oil-in-water emulsion base composed of glycerin, cetyl alcohol, stearic acid, glyceryl monostearate, mineral oil, polyoxyl 40 stearate, menthol, benzyl alcohol, and purified water. Chemical Structure
How Supplied
HOW SUPPLIED Flurandrenolide Lotion USP, 0.05% is supplied in plastic squeeze bottles as follows: 60 mL (NDC 45802- 928 -02) 120 mL (NDC 45802- 928 -03) Keep out of reach of children. Storage: Avoid freezing. Keep tightly closed. Protect from light. Store at 20° to 25°C (68° to 77°F) with excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Rx Only Manufactured By Padagis Yeruham, Israel Distributed By Padagis Allegan, MI 49010 • www.padagis.com Rev 02-22 2U66M RC F2
Indications & Usage
INDICATIONS AND USAGE Flurandrenolide Lotion USP, 0.05% is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
Dosage and Administration
DOSAGE AND ADMINISTRATION Shake well before using. A small quantity of Flurandrenolide Lotion USP, 0.05% should be rubbed gently into the affected area 2 or 3 times daily. Therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary. Flurandrenolide Lotion USP, 0.05% should not be used with occlusive dressings unless directed by a physician. Tight-fitting diapers or plastic pants may constitute occlusive dressings.
