Drug Catalog - Product Detail
FLUVOXAMINE MALEATE ER CP 150MG 30
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00228-2849-03 | ACTAVIS PHARMA | 30 | 150MG | CAPSULE |
PACKAGE FILES



Generic Name
FLUVOXAMINE MALEATE
Substance Name
FLUVOXAMINE MALEATE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA091482
Description
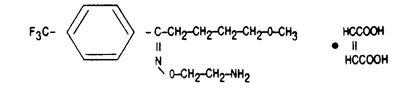
11 DESCRIPTION Fluvoxamine maleate extended-release capsules, for oral administration, contain fluvoxamine maleate USP, a selective serotonin (5-HT) reuptake inhibitor (SSRI) belonging to the chemical series, the 2-aminoethyl oxime ethers of aralkylketones. Fluvoxamine maleate, USP is chemically designated as 5-methoxy-4'-(trifluoromethyl) valerophenone-(E)-O-(2-aminoethyl)oxime maleate (1:1) and has the molecular formula C 15 H 21 O 2 N 2 F 3 •C 4 H 4 O 4 . Its molecular weight is 434.41. The structural formula is: Fluvoxamine maleate, USP is a white to off-white, odorless, crystalline powder that is sparingly soluble in water, freely soluble in ethanol and chloroform, and practically insoluble in diethyl ether. Fluvoxamine maleate extended-release capsules are available in 100 mg and 150 mg strengths for oral administration. In addition to the active ingredient, fluvoxamine maleate USP, each capsule contains the following inactive ingredients: ammonio methacrylate copolymer, type A, ammonio methacrylate copolymer, type B, black iron oxide, FD&C Blue #1, gelatin, hydroxypropyl cellulose, sodium lauryl sulfate, sugar spheres (which contain sucrose and corn starch), talc, titanium dioxide, triethyl citrate, yellow iron oxide. The capsules are imprinted with black Tek-Print ink SW-9008 and SW-9009 which contain black iron oxide, potassium hydroxide, propylene glycol and shellac. ebfb06fd-figure-03
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied Fluvoxamine maleate extended-release capsules are available as follows: 100 mg – Each #2 capsule with olive opaque cap and gray opaque body, imprinted with and 2848 on both cap and body in black ink contains 100 mg of fluvoxamine maleate, USP. Capsules are supplied in bottles of 30 (NDC 0228-2848-03). 150 mg – Each #1 capsule with olive opaque cap and white opaque body, imprinted with and 2849 on both cap and body in black ink contains 150 mg of fluvoxamine maleate, USP. Capsules are supplied in bottles of 30 (NDC 0228-2849-03). ebfb06fd-figure-04 ebfb06fd-figure-05 16.2 Storage Keep out of reach of children. Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from high humidity and avoid exposure to temperatures above 30°C (86°F). Dispense in tight, light-resistant container as defined in the USP.
Indications & Usage
1 INDICATIONS AND USAGE Fluvoxamine maleate extended-release capsules are a selective serotonin reuptake inhibitor (SSRI) indicated for the treatment of obsessive compulsive disorder (OCD) ( 1 ). Efficacy was demonstrated in: One 12-week study with fluvoxamine maleate extended-release capsules in adults ( 14.1 ). Two 10-week studies with immediate-release (IR) fluvoxamine tablets in adults and one 10-week study with IR fluvoxamine tablets in children and adolescents ( 14.1 , 14.3 ). One maintenance study with IR fluvoxamine tablets ( 14.2 ). 1.1 Obsessive Compulsive Disorder Fluvoxamine maleate extended-release capsules are indicated for the treatment of obsessive compulsive disorder (OCD), as defined in the DSM-IV. Obsessive compulsive disorder is characterized by recurrent and persistent ideas, thoughts, impulses, or images (obsessions) that are ego-dystonic and/or repetitive, purposeful, and intentional behaviors (compulsions) that are recognized by the person as excessive or unreasonable. The obsessions or compulsions cause marked distress, are time-consuming, or significantly interfere with social or occupational functioning. The efficacy of fluvoxamine maleate extended-release capsules was demonstrated in one 12-week trial in adults with fluvoxamine maleate extended-release capsules as well as in two 10-week trials in adults and in one 10-week trial in children and adolescents (ages 8 to 17 years) with immediate-release fluvoxamine tablets in outpatients with the diagnosis of OCD as defined in DSM-IV or DSM-III-R (see Clinical Studies [14.1 , 14.3] ) . The efficacy of fluvoxamine for long-term use was established in one maintenance study in adults with immediate-release fluvoxamine tablets (see Clinical Studies [14. 2 ] ) . The health care provider who elects to prescribe fluvoxamine maleate extended-release capsules for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient (see Dosage and Administration [2.4] ) .
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Adults: Recommended starting dose is 100 mg at bedtime, with weekly increases of 50 mg as tolerated to maximum effect, not to exceed 300 mg/day ( 2.1 ). Pediatric patients naïve to fluvoxamine maleate: The lowest available dose of fluvoxamine maleate extended-release capsules may not be appropriate ( 2.2 ). Hepatically impaired: Decreased clearance may require modified dose and titration ( 2.3 ). Extended treatment: Adjust dose to maintain lowest effective dose; reassess patients periodically ( 2.4 ). Discontinuation: Gradual dose reduction is recommended ( 2.7 , see Warnings and Precautions [5.10] ). 2.1 OCD (Obsessive Compulsive Disorder) The recommended starting dose is 100 mg at bedtime, with weekly increases of 50 mg as tolerated to maximum therapeutic benefit, not to exceed 300 mg per day. Capsules should not be crushed or chewed. 2.2 Pediatric Patients Naïve to Fluvoxamine Maleate Physicians should consider that the lowest available dose of fluvoxamine maleate extended-release capsules may not be appropriate for pediatric patients naïve to fluvoxamine maleate. 2.3 Dosage for Elderly or Hepatically Impaired Patients Elderly patients and those with hepatic impairment have been observed to have a decreased clearance of fluvoxamine maleate. Consequently, it may be appropriate to titrate slowly following the initial dose of 100 mg in these patient groups. 2.4 Maintenance/Continuation of Extended Treatment Although the efficacy of fluvoxamine maleate extended-release capsules beyond 12 weeks of dosing has not been documented in controlled trials, OCD is a chronic condition, and it is reasonable to consider continuation for a responding patient. The benefit of maintaining patients with OCD on immediate-release fluvoxamine maleate tablets after achieving a response for an average duration of about 4 weeks in a 10-week single-blind phase during which patients were titrated to effect was demonstrated in a controlled trial (see Clinical Studies [14.2] ) . Dosage adjustments should be made to maintain the patient on the lowest effective dosage, and patients should be periodically reassessed to determine the need for continued treatment. 2.5 Switching a Patient To or From a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with fluvoxamine maleate extended-release capsules. Conversely, at least 14 days should be allowed after stopping fluvoxamine maleate extended-release capsules before starting an MAOI intended to treat psychiatric disorders (see Contraindications [4.1] ) . 2.6 Use of Fluvoxamine Maleate Extended-Release Capsules with Other MAOIs such as Linezolid or Methylene Blue Do not start fluvoxamine maleate extended-release capsules in a patient who is being treated with linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered (see Contraindications [4.1] ). In some cases, a patient already receiving fluvoxamine maleate extended-release capsules therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, fluvoxamine maleate extended-release capsules should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for two weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with fluvoxamine maleate extended-release capsules may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue (see Warnings and Precautions [5.2] ) . The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with fluvoxamine maleate extended-release capsules is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use (see Warnings and Precautions [5.2] ) . 2.7 Discontinuation of Treatment with Fluvoxamine Maleate Extended-Release Capsules Symptoms associated with discontinuation of other SSRIs or SNRIs have been reported (see Warnings and Precautions [5.10] ) . Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the health care provider may continue decreasing the dose but at a more gradual rate.
