Drug Catalog - Product Detail
GALANTAMINE HYDROBROMIDE CAP ER 24HR 16 MG 30 CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 65862-0745-30 | AUROBINDO PHARMA | 30 | 16MG | CAPSULE |
PACKAGE FILES












Generic Name
GALANTAMINE
Substance Name
GALANTAMINE HYDROBROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204895
Description
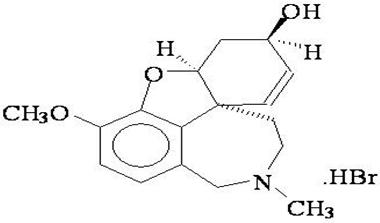
11 DESCRIPTION Galantamine extended-release capsules USP contain galantamine, a reversible, competitive acetylcholinesterase inhibitor, as the hydrobromide salt. Galantamine hydrobromide is known chemically as (4a S ,6 R ,8a S )-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6 H -benzofuro[3a, 3,2- ef ][2]benzazepin-6-ol hydrobromide. It has a molecular formula of C 17 H 21 NO 3 •HBr and a molecular weight of 368.27. Galantamine hydrobromide USP is a white to almost white powder and is sparingly soluble in water. The structural formula for galantamine hydrobromide is: Galantamine extended-release capsules USP contain 8 mg, 16 mg, and 24 mg galantamine as 10.25 mg, 20.50 mg, and 30.76 mg of galantamine hydrobromide USP, respectively. Inactive ingredients include colloidal silicon dioxide, gelatin, hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate, talc, and titanium dioxide. The 16 mg capsule also contains iron oxide red. The 24 mg capsule also contains iron oxide red and iron oxide yellow. The capsules are printed with edible ink containing black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution. Meets USP Dissolution Test 6. Chemical Strucutre
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied Galantamine extended-release capsules USP are supplied as follows: Galantamine Extended-Release Capsules USP, 8 mg are white opaque size “1” hard gelatin capsule with inscription “A” over the cap & “8” over the body containing white to off-white, round, biconvex, single mini tablet. Bottles of 30 NDC 65862-744-30 Bottles of 90 NDC 65862-744-90 Bottles of 100 NDC 65862-744-01 Bottles of 500 NDC 65862-744-05 Bottles of 1,000 NDC 65862-744-99 Galantamine Extended-Release Capsules USP, 16 mg are pink opaque size “1” hard gelatin capsule with inscription “A” over the cap & “16” over the body containing white to off-white, round, biconvex, two mini tablets. Bottles of 30 NDC 65862-745-30 Bottles of 90 NDC 65862-745-90 Bottles of 100 NDC 65862-745-01 Bottles of 500 NDC 65862-745-05 Bottles of 1,000 NDC 65862-745-99 Galantamine Extended-Release Capsules USP, 24 mg are caramel opaque size “1” hard gelatin capsule with inscription “A” over the cap & “24” over the body containing white to off-white, round, biconvex, three mini tablets. Bottles of 30 NDC 65862-746-30 Bottles of 90 NDC 65862-746-90 Bottles of 100 NDC 65862-746-01 Bottles of 500 NDC 65862-746-05 Bottles of 1,000 NDC 65862-746-99 Storage and Handling Galantamine extended-release capsules USP should be stored at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Keep out of reach of children.
Indications & Usage
1 INDICATIONS AND USAGE Galantamine extended-release capsules are indicated for the treatment of mild to moderate dementia of the Alzheimer’s type. Galantamine extended-release capsules are a cholinesterase inhibitor indicated for the treatment of mild to moderate dementia of the Alzheimer’s type (1)
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Recommended starting dosage is 8 mg/day in morning; increase to initial maintenance dose of 16 mg/day after a minimum of 4 weeks. Based on clinical benefit and tolerability, dosage may be increased to 24 mg/day after a minimum of 4 weeks at 16 mg/day. ( 2.1 ) Take with food; ensure adequate fluid intake during treatment (2.1) Hepatic impairment: should not exceed 16 mg/day for moderate hepatic impairment; do not use in patients with severe hepatic impairment ( 2.2 ) Renal impairment: should not exceed 16 mg/day for creatinine clearance 9 to 59 mL/min; do not use in patients with creatinine clearance less than 9 mL/min. ( 2.3 ) Conversion from galantamine tablets to galantamine extended-release capsules should occur at the same daily dosage with the last dose of galantamine tablets taken in evening and starting galantamine extended-release capsules once daily treatment the next morning. ( 2.5 ) 2.1 Recommended Dosage and Administration Administer galantamine extended-release capsules once daily in the morning, preferably with food. Ensure adequate fluid intake during treatment. The recommended starting dosage of galantamine extended-release capsules is 8 mg/day. Increase to the initial maintenance dosage of 16 mg/day after a minimum of 4 weeks. A further increase to 24 mg/day may be attempted after a minimum of 4 weeks at 16 mg/day. Increase dosage based upon assessment of clinical benefit and tolerability of the previous dosage. The dosage of galantamine extended-release capsules shown to be effective in a controlled clinical trial is 16 to 24 mg/day. 2.2 Dosage in Patients with Hepatic Impairment In patients with moderate hepatic impairment (Child-Pugh score of 7 to 9), the dosage should generally not exceed 16 mg/day. The use of galantamine extended-release capsules in patients with severe hepatic impairment (Child-Pugh score of 10 to 15) is not recommended [see Clinical Pharmacology (12.3) ] . 2.3 Dosage in Patients with Renal Impairment In patients with creatinine clearance of 9 to 59 mL/min, the dosage should generally not exceed 16 mg/day. In patients with creatinine clearance less than 9 mL/min, the use of galantamine extended-release capsules is not recommended [see Clinical Pharmacology (12.3) ] . 2.4 Treatment Interruption If therapy has been interrupted for more than three days, the patient should be restarted at the lowest dosage and the dosage escalated to the current dose. The abrupt withdrawal of galantamine extended-release capsules in those patients who had been receiving dosages in the effective range was not associated with an increased frequency of adverse events in comparison with those continuing to receive the same dosages of that drug. 2.5 Switching to Galantamine Extended-Release Capsules from Galantamine Tablets Patients currently being treated with galantamine tablets can convert to galantamine extended-release capsules by taking their last dose of galantamine tablets in the evening and starting galantamine extended-release capsules once daily treatment the next morning. Converting from galantamine tablets to galantamine extended-release capsules should occur at the same total daily dosage.
