Drug Catalog - Product Detail
GALANTAMINE HYDROBROMIDE ER 24MG CP 30CT
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00591-3498-30 | ACTAVIS PHARMA | 30 | 24MG | CAPSULE |
PACKAGE FILES


Generic Name
GALANTAMINE HYDROBROMIDE
Substance Name
GALANTAMINE HYDROBROMIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA079028
Description
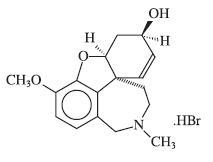
DESCRIPTION Galantamine hydrobromide is a reversible, competitive acetylcholinesterase inhibitor. Galantamine hydrobromide is known chemically as (4aS,6R,8aS)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro[3a,3,2-ef][2]benzazepin-6-ol hydrobromide. It has a molecular formula of C 17 H 21 NO 3 •HBr and a molecular weight of 368.27. Galantamine hydrobromide is a white to almost white powder and is sparingly soluble in water. The structural formula for galantamine hydrobromide is: Galantamine hydrobromide extended-release capsules are available in opaque hard gelatin extended-release capsules of 8 mg (white), 16 mg (pink and white), and 24 mg (pink) containing galantamine hydrobromide, equivalent to respectively 8, 16 and 24 mg galantamine base. Inactive ingredients include gelatin, hydroxypropyl cellulose, magnesium stearate, colloidal silicon dioxide and titanium dioxide. The 16 mg capsule and 24 mg capsule also contain red ferric oxide.
How Supplied
HOW SUPPLIED Galantamine hydrobromide extended-release capsules contain white to off-white matrix tablets. 8 mg white opaque cap and body, size 1 hard gelatin capsules with inscription “WPI 3496” 16 mg pink opaque cap and white opaque body, size 1 hard gelatin capsules with the inscription “WPI 3497” 24 mg pink opaque cap and body, size 1 hard gelatin capsules with the inscription “WPI 3498” The capsules are supplied as follows: 8 mg capsules – bottles of 30 NDC 0591-3496-30 8 mg capsules – bottles of 500 NDC 0591-3496-05 16 mg capsules – bottles of 30 NDC 0591-3497-30 16 mg capsules – bottles of 500 NDC 0591-3497-05 24 mg capsules – bottles of 30 NDC 0591-3498-30 24 mg capsules – bottles of 500 NDC 0591-3498-05 Storage and Handling Galantamine hydrobromide extended-release capsules should be stored at 20° to 25°C (68°-77°F). [See USP controlled room temperature.] Keep out of reach of children. Galantamine hydrobromide extended-release capsules are manufactured by: Watson Laboratories, Inc. Corona, CA 92880 USA Distributed by: Watson Pharma, Inc. Corona, CA 92880 USA Issued: June 2008 173479 0608B
Indications & Usage
INDICATIONS AND USAGE Galantamine hydrobromide extended-release capsules are indicated for the treatment of mild to moderate dementia of the Alzheimer’s type.
Dosage and Administration
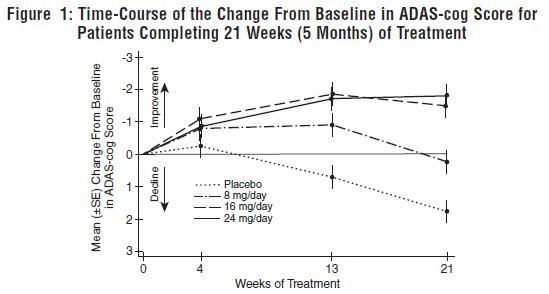
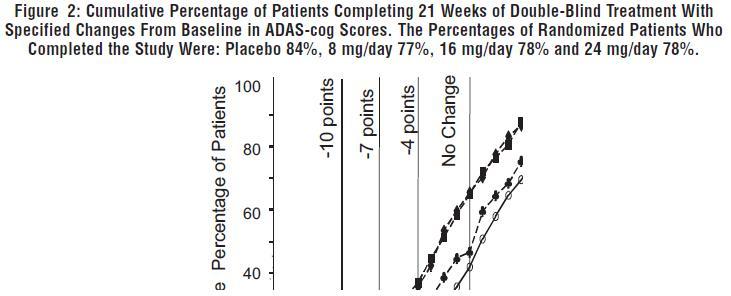
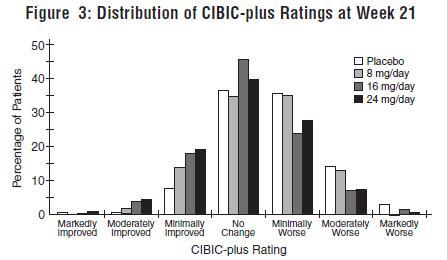
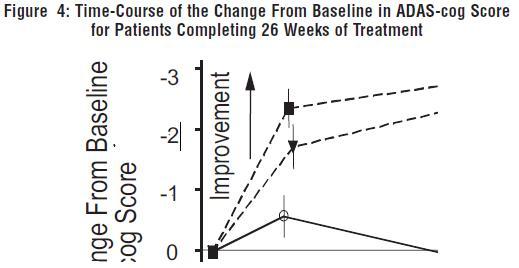
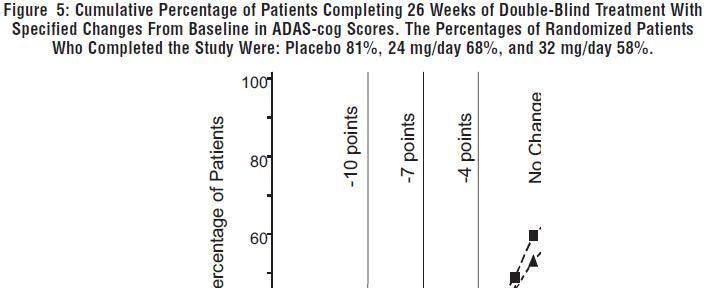
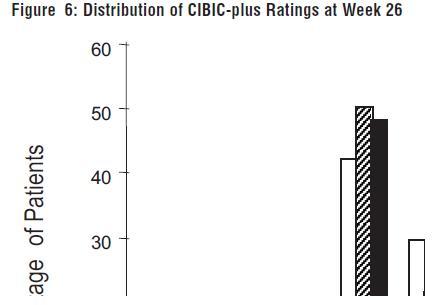
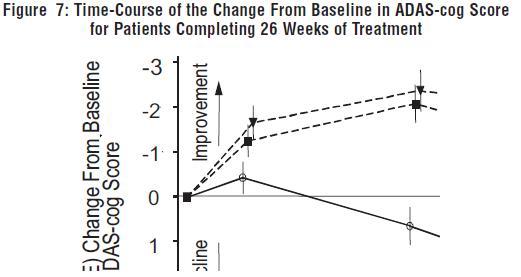
DOSAGE AND ADMINISTRATION Galantamine Hydrobromide Extended-Release Capsules The dosage of galantamine hydrobromide extended-release capsules shown to be effective in a controlled clinical trial is 16-24 mg/day. The recommended starting dose of galantamine hydrobromide extended-release capsules is 8 mg/day. The dose should be increased to the initial maintenance dose of 16 mg/day after a minimum of 4 weeks. A further increase to 24 mg/day should be attempted after a minimum of 4 weeks at 16 mg/day. Dose increases should be based upon assessment of clinical benefit and tolerability of the previous dose. Galantamine hydrobromide extended-release capsules should be administered once daily in the morning, preferably with food. Patients currently being treated with galantamine hydrobromide immediate-release tablets can convert to galantamine hydrobromide extended-release capsules by taking their last dose of galantamine hydrobromide immediate-release tablets in the evening and starting galantamine hydrobromide extended-release capsules once daily treatment the next morning. Converting from galantamine hydrobromide immediate-release tablets to galantamine hydrobromide extended-release capsules should occur at the same total daily dose. Patients and caregivers should be advised to ensure adequate fluid intake during treatment. If therapy has been interrupted for several days or longer, the patient should be restarted at the lowest dose and the dose escalated to the current dose. The abrupt withdrawal of galantamine hydrobromide extended-release capsules in those patients who had been receiving doses in the effective range was not associated with an increased frequency of adverse events in comparison with those continuing to receive the same doses of that drug. The beneficial effects of galantamine hydrobromide extended-release capsules are lost, however, when the drug is discontinued. Doses in Special Populations Galantamine plasma concentrations may be increased in patients with moderate to severe hepatic impairment. In patients with moderately impaired hepatic function (Child-Pugh score of 7-9), the dose should generally not exceed 16 mg/day. The use of galantamine hydrobromide extended-release capsules in patients with severe hepatic impairment (Child-Pugh score of 10-15) is not recommended. For patients with moderate renal impairment the dose should generally not exceed 16 mg/day. In patients with severe renal impairment (creatinine clearance < 9 mL/min), the use of galantamine hydrobromide extendedrelease capsules are not recommended.
