Drug Catalog - Product Detail
GLIPIZIDE ER XL 5MG TB 100
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 64980-0280-01 | RISING PHARMACEUTICALS | 100 | 5MG | TABLET |
PACKAGE FILES



Generic Name
GLIPIZIDE
Substance Name
GLIPIZIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA204720
Description
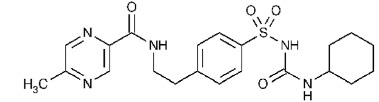
11 DESCRIPTION Glipizide extended-release tablets contain glipizide which is an oral sulfonylurea. The Chemical Abstracts name of glipizide is 1-cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido) ethyl] phenyl]sulfonyl]urea. The molecular formula is C 21 H 27 N 5 O 4 S; the molecular weight is 445.55; the structural formula is shown below Glipizide is a whitish, odorless powder with a pKa of 5.9. It is insoluble in water and alcohols, but s oluble in 0.1 N NaOH; it is freely soluble in dimethylformamide. Each extended-release film-coated tablet contains 2.63 mg glipizide, USP to provide a 2.5 mg dose. Each extended-release film-coated tablet contains 5.25 mg glipizide, USP to provide a 5 mg dose. Each extended-release film-coated tablet contains 10.50 mg glipizide, USP to provide a 10 mg dose. Inert ingredients in the 2.5 mg, 5 mg and 10 mg formulations are: cellulose acetate, ferrosoferric oxide, hypromellose, magnesium stearate, polyethylene glycol, polyethylene oxide, propylene glycol, red ferric oxide, shellac, sodium chloride, titanium dioxide. The 2.5 mg tablet also contains: FD&C blue#2, lactose monohydrate and triacetin. System Components and Performance Glipizide extended-release tablets are similar in appearance to a conventional tablet. It consists, however, of an osmotically active drug core surrounded by a semipermeable membrane. The core itself is divided into two layers: an “active” layer containing the drug, and a “push” layer containing pharmacologically inert (but osmotically active) components. The membrane surrounding the tablet is permeable to water but not to drug or osmotic excipients. As water from the gastrointestinal tract enters the tablet, pressure increases in the osmotic layer and “pushes” against the drug layer, resulting in the release of drug through a small, laser-drilled orifice in the membrane on the drug side of the tablet. The function of the glipizide extended-release tablets depends upon the existence of an osmotic gradient between the contents of the bi-layer core and fluid in the GI tract. The biologically inert components of the tablet remain intact during GI transit and are eliminated in the feces as an insoluble shell. structural formula
How Supplied
16 HOW SUPPLIED/STORAGE AND HANDLING Glipizide extended-release tablets are supplied to provide 2.5 mg, 5 mg, and 10 mg round, biconvex tablets and imprinted with black ink as follows: Table 2: Glipizide extended-release tablet Presentations Tablet Strength Tablet Color/ Shape Tablet Markings Package Size NDC Code 2.5 mg Blue Round Biconvex imprinted with “P ” on one side. 2.5 Bottles of 30 NDC 64980-279-03 5 mg White Round Biconvex imprinted with “P ” on one side. 5 Bottles of 100 NDC 64980-280-01 imprinted with “P ” on one side. 5 Bottles of 500 NDC 64980-280-05 imprinted with “P ” on one side. 5 Bottles of 1000 NDC 64980-280-10 10 mg White Round Biconvex imprinted with “P ” on one side. 10 Bottles of 100 NDC 64980-281-01 imprinted with “P ” on one side. 10 Bottles of 500 NDC 64980-281-05 imprinted with “P ” on one side. 10 Bottles of 1000 NDC 64980-281-10 Recommended Storage: The tablets should be protected from moisture and humidity. Store at 20° to 25°C (68° to 77º F); [see USP Controlled Room Temperature].
Indications & Usage
1 INDICATIONS AND USAGE Glipizide extended-release tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Glipizide extended-release tablet is a sulfonylurea indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus Limitations of Use: Not for treatment of type 1 diabetes or diabetic ketoacidosis 1.1 Limitations of Use Glipizide extended-release tablets are not recommended for the treatment of type 1 diabetes mellitus or diabetic ketoacidosis.
Dosage and Administration
2 DOSAGE AND ADMINISTRATION Recommended starting dose is 5 mg once daily. Dose adjustment can be made based on the patient’s glycemic control. Maximum recommended dose is 20 mg once daily ( 2.1 ). Administer with breakfast or the first meal of the day ( 2.1 ). For combination therapy with other blood-glucose-lowering agents, initiate the agent at the lowest recommended dose, and observe patients for hypoglycemia ( 2.2 ). 2.1 Recommended Dosing Glipizide extended-release tablets should be administered orally with breakfast or the first main meal of the day. The recommended starting dose of glipizide extended-release tablets is 5 mg once daily. Start patients at increased risk for hypoglycemia (e.g. the elderly or patients with hepatic insufficiency) at 2.5 mg [see Use in Specific Population (8.5 , 8.6) ] . Dosage adjustment can be made based on the patient’s glycemic control. The maximum recommended dose is 20 mg once daily. Patients receiving immediate release glipizide may be switched to glipizide extended-release tablets once daily at the nearest equivalent total daily dose. 2.2 Use with Other Glucose Lowering Agents When adding glipizide extended-release tablets to other anti-diabetic drugs, initiate glipizide extended-release tablets at 5 mg once daily. Start patients at increased risk for hypoglycemia at a lower dose. When colesevelam is coadministered with glipizide extended-release tablets, maximum plasma concentration and total exposure to glipizide is reduced. Therefore, glipizide extended-release tablets should be administered at least 4 hours prior to colesevelam.
