Drug Catalog - Product Detail
Glipizide-Metformin HCl Tab 2.5-500 MG 100 EA
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 68382-0185-01 | ZYDUS PHARMACEUTICALS (USA) | 100 | 2.5-500MG | TABLET |
PACKAGE FILES



Generic Name
GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Substance Name
GLIPIZIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
ORAL
Application Number
ANDA078905
Description
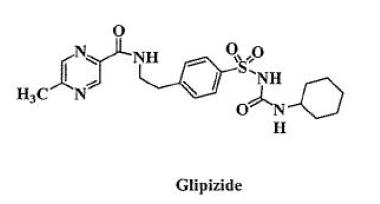
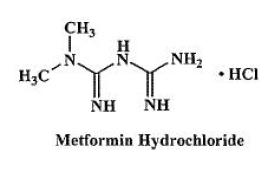
DESCRIPTION Glipizide and metformin hydrochloride tablets contain two oral antihyperglycemic drugs used in the management of type 2 diabetes, glipizide and metformin hydrochloride. Glipizide is an oral antihyperglycemic drug of the sulfonylurea class. The chemical name for glipizide is 1-cyclohexyl-3-[[ p -[2-(5-methylpyrazinecarboxamido)ethyl]phenyl] sulfonyl]urea. Glipizide, USP is a white to almost white; crystalline powder with a molecular formula of C 21 H 27 N 5 O 4 S, a molecular weight of 445.55 and a pK a of 5.9. The structural formula is represented below. Metformin hydrochloride, USP is an oral antihyperglycemic drug used in the management of type 2 diabetes. Metformin hydrochloride ( N,N -dimethylimidodicarbonimidic diamide monohydrochloride) is not chemically or pharmacologically related to sulfonylureas, thiazolidinediones, or α-glucosidase inhibitors. It is white crystalline compound with a molecular formula of C 4 H 12 ClN 5 (monohydrochloride) and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol, practically insoluble in acetone and in methylene chloride. The pK a of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is as shown: Each glipizide and metformin hydrochloride tablet intended for oral administration contains glipizide, 2.5 mg or 5 mg and metformin hydrochloride, 250 mg or 500 mg. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone and titanium dioxide. Additionally each 2.5 mg/250 mg and 5 mg/500 mg tablet contains iron oxide red and each 2.5 mg/500 mg tablet contains polysorbate 80. structuaral formula 01 structural formula 02
How Supplied
HOW SUPPLIED Glipizide and Metformin Hydrochloride Tablets, 2.5 mg/250 mg are pink-colored, biconvex, modified capsule-shaped, film-coated tablet, debossed with "ZE68" on one side and plain on other side and are supplied as follows: NDC 68382-184-16 in bottle of 90 tablets with child-resistant closure NDC 68382-184-01 in bottle of 100 tablets NDC 68382-184-10 in bottle of 1000 tablets NDC 68382-184-77 in unit-dose blister cartons of 100 (10 x 10) unit-dose tablets Glipizide and Metformin Hydrochloride Tablets, 2.5 mg/500 mg are white-colored, biconvex, modified capsule-shaped, film-coated tablet, debossed with "ZE67" on one side and plain on other side and are supplied as follows: NDC 68382-185-16 in bottle of 90 tablets with child-resistant closure NDC 68382-185-01 in bottle of 100 tablets NDC 68382-185-10 in bottle of 1000 tablets NDC 68382-185-77 in unit-dose blister cartons of 100 (10 x 10) unit-dose tablets Glipizide and Metformin Hydrochloride Tablets, 5 mg/500 mg are pink-colored, biconvex, modified capsule-shaped, film-coated tablet, debossed with "ZE66" on one side and plain on other side and are supplied as follows: NDC 68382-186-16 in bottle of 90 tablets with child-resistant closure NDC 68382-186-01 in bottle of 100 tablets NDC 68382-186-10 in bottle of 1000 tablets NDC 68382-186-77 in unit-dose blister cartons of 100 (10 x 10) unit-dose tablets
Indications & Usage
INDICATIONS AND USAGE Glipizide and metformin hydrochloride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Dosage and Administration
DOSAGE AND ADMINISTRATION General Considerations: Dosage of glipizide and metformin hydrochloride tablets must be individualized on the basis of both effectiveness and tolerance while not exceeding the maximum recommended daily dose of 20 mg glipizide/2000 mg metformin. Glipizide and metformin hydrochloride tablets should be given with meals and should be initiated at a low dose, with gradual dose escalation as described below, in order to avoid hypoglycemia (largely due to glipizide), to reduce GI side effects (largely due to metformin), and to permit determination of the minimum effective dose for adequate control of blood glucose for the individual patient. With initial treatment and during dose titration, appropriate blood glucose monitoring should be used to determine the therapeutic response to glipizide and metformin hydrochloride tablets and to identify the minimum effective dose for the patient. Thereafter, HbA 1c should be measured at intervals of approximately 3 months to assess the effectiveness of therapy. The therapeutic goal in all patients with type 2 diabetes is to decrease FPG, PPG, and HbA 1c to normal or as near normal as possible. Ideally, the response to therapy should be evaluated using HbA 1c (glycosylated hemoglobin), which is a better indicator of long-term glycemic control than FPG alone. No studies have been performed specifically examining the safety and efficacy of switching to glipizide and metformin hydrochloride tablets therapy in patients taking concomitant glipizide (or other sulfonylurea) plus metformin. Changes in glycemic control may occur in such patients, with either hyperglycemia or hypoglycemia possible. Any change in therapy of type 2 diabetes should be undertaken with care and appropriate monitoring. When colesevelam is coadministered with glipizide ER, maximum plasma concentration and total exposure to glipizide is reduced. Therefore, glipizide and metformin hydrochloride tablets should be administered at least 4 hours prior to colesevelam. Glipizide and Metformin Hydrochloride Tablets in Patients with Inadequate Glycemic Control on Diet and Exercise Alone For patients with type 2 diabetes whose hyperglycemia cannot be satisfactorily managed with diet and exercise alone, the recommended starting dose of glipizide and metformin hydrochloride tablets is 2.5 mg/250 mg once a day with a meal. For patients whose FPG is 280 mg/dL to 320 mg/dL a starting dose of glipizide and metformin hydrochloride tablets 2.5 mg/500 mg twice daily should be considered. The efficacy of glipizide and metformin hydrochloride tablets in patients whose FPG exceeds 320 mg/dL has not been established. Dosage increases to achieve adequate glycemic control should be made in increments of one tablet per day every two weeks up to maximum of 10 mg/1000 mg or 10 mg/2000 mg glipizide and metformin hydrochloride tablets per day given in divided doses. In clinical trials of glipizide and metformin hydrochloride tablets as initial therapy, there was no experience with total daily doses greater than 10 mg/2000 mg per day. Glipizide and Metformin Hydrochloride Tablets in Patients with Inadequate Glycemic Control on a Sulfonylurea and/or Metformin For patients not adequately controlled on either glipizide (or another sulfonylurea) or metformin alone, the recommended starting dose of glipizide and metformin hydrochloride tablets is 2.5 mg/500 mg or 5 mg/500 mg twice daily with the morning and evening meals. In order to avoid hypoglycemia, the starting dose of glipizide and metformin hydrochloride tablets should not exceed the daily doses of glipizide or metformin already being taken. The daily dose should be titrated in increments of no more than 5 mg/500 mg up to the minimum effective dose to achieve adequate control of blood glucose or to a maximum dose of 20 mg/2000 mg per day. Patients previously treated with combination therapy of glipizide (or another sulfonylurea) plus metformin may be switched to glipizide and metformin hydrochloride tablets 2.5 mg/500 mg or 5 mg/500 mg; the starting dose should not exceed the daily dose of glipizide (or equivalent dose of another sulfonylurea) and metformin already being taken. The decision to switch to the nearest equivalent dose or to titrate should be based on clinical judgment. Patients should be monitored closely for signs and symptoms of hypoglycemia following such a switch and the dose of glipizide and metformin hydrochloride tablets should be titrated as described above to achieve adequate control of blood glucose. Recommendations for Use in Renal Impairment Assess renal function prior to initiation of glipizide and metformin hydrochloride and periodically thereafter. Glipizide and metformin hydrochloride is contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/minute/1.73 m 2 . Initiation of glipizide and metformin hydrochloride in patients with an eGFR between 30 – 45 mL/minute/1.73 m 2 is not recommended. In patients taking glipizide and metformin hydrochloride whose eGFR later falls below 45 mL/min/1.73 m 2 , assess the benefit risk of continuing therapy. Discontinue glipizide and metformin hydrochloride if the patient's eGFR later falls below 30 mL/minute/1.73 m 2 . (See WARNINGS.) Discontinuation for Iodinated Contrast Imaging Procedures Discontinue glipizide and metformin hydrochloride at the time of, or prior to, an iodinated contrast imaging procedure in patients with an eGFR between 30 and 60 mL/min/1.73 m 2 ; in patients with a history of liver disease, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart glipizide and metformin hydrochloride if renal function is stable. Specific Patient Populations: Glipizide and metformin hydrochloride tablets are not recommended for use during pregnancy or for use in pediatric patients. The initial and maintenance dosing of glipizide and metformin hydrochloride tablets should be conservative in patients with advanced age, due to the potential for decreased renal function in this population. Any dosage adjustment requires a careful assessment of renal function. Generally, elderly, debilitated, and malnourished patients should not be titrated to the maximum dose of glipizide and metformin hydrochloride tablets to avoid the risk of hypoglycemia. Monitoring of renal function is necessary to aid in prevention of metformin-associated lactic acidosis, particularly in the elderly (see WARNINGS and PRECAUTIONS ).
