Drug Catalog - Product Detail
HALCINONIDE TOP APP CREAM 0.1% 60GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 00378-8056-60 | MYLAN | 60 | 0.1% | CREAM |
PACKAGE FILES
Generic Name
HALCINONIDE
Substance Name
HALCINONIDE
Product Type
HUMAN PRESCRIPTION DRUG
Route
TOPICAL
Application Number
ANDA211027
Description
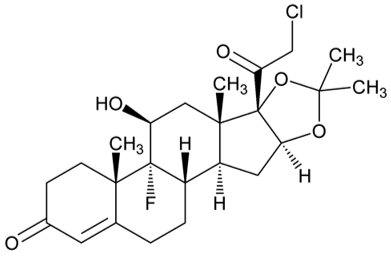
DESCRIPTION The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. The steroids in this class include halcinonide. Halcinonide is designated chemically as 21-Chloro-9-fluoro-11β,16α,17-trihydroxypregn-4-ene-3,20-dione cyclic 16,17-acetal with acetone. Graphic formula: C 24 H 32 C1FO 5 MW 454.96 Each gram of 0.1% halcinonide cream, USP contains 1 mg halcinonide, USP in a specially formulated cream base consisting of cetyl alcohol, dimethicone 360, glyceryl stearate, isopropyl palmitate, nitrogen, polysorbate 60, propylene glycol, purified water, and titanium dioxide. Halcinonide Structural Formula
How Supplied
HOW SUPPLIED Halcinonide Cream, USP 0.1% contains halcinonide, USP. The white to off-white cream is available as follows: NDC 0378-8056-49 carton containing one 30 g tube NDC 0378-8056-60 carton containing one 60 g tube Storage: Store at 20ºC to 25ºC (68ºF to 77ºF). [See USP Controlled Room Temperature.] Avoid excessive heat (104°F). To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Manufactured for: Mylan Pharmaceuticals Inc. Morgantown, WV 26505 U.S.A. Manufactured by: DPT Laboratories, Ltd. San Antonio, TX 78215 U.S.A. 141101-0819 Revised: 8/2019 DPT:HALCCR:R2
Indications & Usage
INDICATIONS AND USAGE Halcinonide cream, 0.1% is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
Dosage and Administration
DOSAGE AND ADMINISTRATION Apply the 0.1% halcinonide cream to the affected area two to three times daily. Rub in gently. Occlusive Dressing Technique Occlusive dressings may be used for the management of psoriasis or other recalcitrant conditions. Gently rub a small amount of cream into the lesion until it disappears. Reapply the preparation leaving a thin coating on the lesion, cover with a pliable nonporous film, and seal the edges. If needed, additional moisture may be provided by covering the lesion with a dampened clean cotton cloth before the nonporous film is applied or by briefly wetting the affected area with water immediately prior to applying the medication. The frequency of changing dressings is best determined on an individual basis. It may be convenient to apply halcinonide cream under an occlusive dressing in the evening and to remove the dressing in the morning (i.e., 12-hour occlusion). When utilizing the 12-hour occlusion regimen, additional cream should be applied, without occlusion, during the day. Reapplication is essential at each dressing change. If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
