Drug Catalog - Product Detail
HALOBETASOL PROPIONATE OINTMENT OINT 0.0005 15GM
| NDC | Mfr | Size | Str | Form |
|---|---|---|---|---|
| 45802-0131-35 | PADAGIS | 15 | 0.05% | OINTMENT |
PACKAGE FILES
Generic Name
Substance Name
Product Type
Route
Application Number
Description
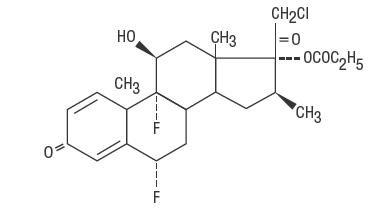
DESCRIPTION Halobetasol propionate ointment, 0.05% contains halobetasol propionate, a synthetic corticosteroid for topical dermatological use. The corticosteroids constitute a class of primarily synthetic steroids used topically as an anti-inflammatory and antipruritic agent. Chemically halobetasol propionate is 21-chloro-6α, 9-difluoro-11β, 17-dihydroxy-16β-methylpregna-1, 4-diene-3-20-dione, 17-propionate, C 25 H 31 ClF 2 O 5 . It has the following structural formula: Halobetasol propionate has the molecular weight of 485. It is a white crystalline powder insoluble in water. Each gram of halobetasol propionate ointment, 0.05% contains 0.5 mg of halobetasol propionate in a base of aluminum stearate, beeswax, pentaerythritol cocoate, stearyl citrate, petrolatum, propylene glycol and sorbitan sesquioleate. structural formula
How Supplied
HOW SUPPLIED Halobetasol propionate ointment, 0.05% is supplied in the following tube sizes: 15 g (NDC 45802-131-35) and 50 g (NDC 45802-131-32) STORAGE Store between 15°C and 30°C (59°F and 86°F).
Indications & Usage
INDICATIONS AND USAGE Halobetasol propionate ointment, 0.05% is a super-high potency corticosteroid indictated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Treatment beyond two consecutive weeks is not recommended, and the total dosage should not exceed 50 g/week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis.
Dosage and Administration
DOSAGE AND ADMINISTRATION Apply a thin layer of halobetasol propionate ointment, 0.05% to the affected skin once or twice daily, as directed by your physician, and rub in gently and completely. Halobetasol propionate ointment, 0.05% is a high potency topical corticosteroid; therefore, treatment should be limited to two weeks, and amounts greater than 50g/wk should not be used. As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary. Halobetasol propionate ointment, 0.05% should not be used with occlusive dressings.
